Atmospheric Pollution Exe-8B Chemistry Class-9 ICSE Selina Publishers Solutions Chapter-8. Step By Step ICSE Selina Concise Solutions of Chapter-8 Atmospheric Pollution with All Exercise including MCQs, Very Short Answer Type, Short Answer Type, Long Answer Type, Numerical and Structured/Application Questions Solved . Visit official Website CISCE for detail information about ICSE Board Class-9.
Atmospheric Pollution Exe-8B Chemistry Class-9 ICSE Concise Selina Publishers
| Board | ICSE |
| Publications | Selina Publication |
| Subject | Chemistry |
| Class | 9th |
| Chapter-8 | Atmospheric Pollution |
| Book Name | Concise |
| Topics | Solution of Exercise – 8B (Acid Rain) |
| Academic Session | 2023-2024 |
B. Exercise – 8B
Atmospheric Pollution Class-9 Chemistry Concise Solutions
Page-140
Question 1.
Why does rain water have pH less than 7?
Answer:
Carbon dioxide reacts with water to form weak carbonic acid which is slightly acidic having pH about 5.6.
Hence, the pH of rain water usually ranges between 5.6 and 3.5; at times, it can be as low as 2.
Question 2.
pH of acid rain is sometimes as low as 2. Explain.
Answer:
Normal rain is only slightly acidic having pH about 5.6.
This is because carbon dioxide reacts with it to form weak carbonic acid.
CO2 + H2O → H2CO3
pH of acid rain usually ranges between 5.6 and 3.5; at times, it can be as low as 2.
Question 3.
Explain the formation of acid rain due to:
(i) Oxides of sulphur
(ii) Oxides of nitrogen
Answer:
(i) Oxides of sulphur:
Sulphur is a non-metallic element found in coal and fuel oil.
When these fuels are burned, sulphur combines with oxygen in the air to form its gaseous oxides, sulphur dioxide (SO2) and sulphur trioxide (SO3).
S + O2 → SO2
2SO2 + O2 → 2SO3
Sulphur dioxide and sulphur trioxide react with water to form H2SO4 which is the main cause of acid rain.
2SO2 + O2 + 2H2O →2H2SO4
SO3 + H2O →H2SO4
(ii) Oxides of nitrogen:
Nitric acid is formed by the combination of nitrogen and oxygen. Nitrogen and oxygen combine in the presence of thunder and lightning. Oxides of nitrogen are also produced by internal combustion engines.
N2 + O2 → 2NO
Nitrogen oxide then gets oxidised in the atmosphere to nitrogen dioxide.
2NO + O2 → 2NO2
Nitrogen dioxide combines with water to form a mixture of nitrous acid and nitric acid.
2NO2 + H2O → HNO2 + HNO3
Question 4.
What are the causes of acid rain?
Answer:
The main causes of acid rain are the formation of mineral acids such as carbonic acid, nitric acid andsulphuric acid during rains.
Question 5.
Give the impact of acid rain:
(i) on plants
(i) on soil
(iii) on water bodies
Answer:
(i) Acid rain causes loss of nutrients from plants, thus damaging their leaves.
(ii) It removes calcium and potassium (elements of soil), thus making it lose its fertility which ultimately damages forests.
(iii) Acid rain has serious ecological impacts as it affects water bodies too. Water in lakes and rivers is gradually becoming acidic due to acid rain. This adversely affects aquatic life.
Question 6.
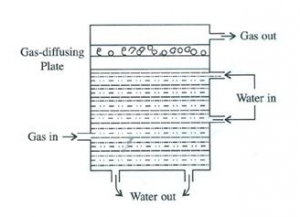
How does a scrubber help in reducing the formation of acid rain?
Answer:
A scrubber can also be used to reduce the formation of acid rain. It is a device which absorbs gaseous pollutants. It is used for removing sulphur dioxide from a smoke stack, and usually consists of a fine spray of water and gas rising from the stack, which is passed through the scrubber where water absorbs sulphur dioxide.
— : End of Atmospheric Pollution Exe-8B Answer Class-9 ICSE Chemistry Solutions :–
Return to Return to Concise Selina ICSE Chemistry Class-9
Thanks
Please share with your friends