Atomic Structure Exe-1 Goyal Brother Solutions ICSE Class-9 Ch-4. We Provide Solutions of Chapter-4 Exercise-1 Atomic Structure and Chemical Bonding Goyal Brother Prakashan ICSE Class-9 Ch-4. Visit official Website CISCE for detail information about ICSE Board Class-9.
Atomic Structure Exe-1 Goyal Brother Solutions ICSE Class-9 Ch-4
| Board | ICSE |
| Publications | Goyal Brother Prakashan |
| Subject | Chemistry |
| Class | 9th |
| Writer | Dr. S.K. Aggarwal |
| Chapter-4 | Atomic Structure and Chemical Bonding |
| Topics | Solutions of Exe-1 |
| Edition | for 2022-2023 Academic Session |
Atomic Structure Exe-1 Goyal Brother Solutions
ICSE Class-9 Chemistry Ch-4
(Page-93 – 95)
Questions 1. Name the Indian Sage who first propounded the idea of atom (anu).
Answer : Acharya Kanad
Questions 2. Draw a labelled diagram to show how cathode rays are produced.
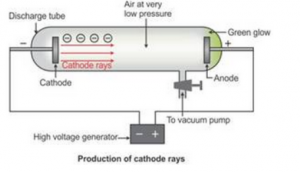
Questions 3. What is an electron State is charge in coulombs.
Answer : electric charge, equal to 1.602176634 × 10−19 coulomb
Questions 4. How many electrons will weigh as much as one atom of hydrogen?
Answer : 1837 electrons.
Questions 5. Who discovered positively charged rays?
Answer : Positively charged radiation was discovered by Goldstein in 1886. These are called canal rays.
Questions 6. Does the mass and charge on positive rays remain same with the change in nature of gas in a discharge tube?
Answer : charge and mass is constant no matter what gas you use in the discharge tube. So, their charge/mass ratio remains constant
Questions 7. Name a gas which produces positive rays which only consists of protons.
Answer : Hydrogen gas
Questions 8. What is a proton? State the charge on a proton in coulombs and mass in grams.
Answer : Proton is the positively charged particle present in the nucleus of the atom. Magnitude of charge: Charge of proton is 1.6022 x 10-19 coulomb. Mass of proton: Mass of proton is 1.0072766 a.m.u. or 1.6726 x 10–27 kg.
Questions 9. How does the mass of a proton compare with the mass of
(a) an atom of hydrogen –The mass of a proton was calculated as being equal to the mass of the hydrogen atom.
(b) an electron–approximately 1837 times that of the electron.
Questions 10. State three properties of positive rays.
Answer : three properties of positive rays
- i) They travel in straight lines and cast shadows of objects placed in their path. …
- (ii) They are deflected by electric and magnetic fields but the deflections are small as compared to that of cathode rays.
- iii) They are the streams of positive ions of the gas enclosed in the discharge tube.
Questions 11 . Whenever a gas is subjected to electric discharge at low pressure, cathode rays and positive rays formed. what does the formation of these rays tell us about the nature of atoms in he gas?
Answer : these are fundamental particles
Questions 12. State three ways by which a proton differs from an electron.
Answer : three ways by which a proton differs from an electron.
| Electron | Proton |
|---|---|
| It revolves in fixed orbits around the nucleus. | It is found inside the nucleus of an atom. |
| It requires less amount of energy to be removed from the atom. | It requires a lot of energy to be removed from the atom. |
| It is represented by the symbol “e”. | It is represented by the symbol “p” |
Questions 13. Fill in the blank spaces with appropriate words:
(a) The charge on the positive rays depends upon ….Nature… of gas in the ..discharge tube .. tube
(b) The anode rays (positive rays) obtained from ….All.. gas, Consist of Only protons.
Questions 14. Briefly describe Thomson’s atomic model. Why was this model not accepted?
Answer : an atom is made up of a positively charged sphere into which negatively charged electrons are implanted. Because electrons and protons have the same magnitude, an atom as a whole is electrically neutral
Atomic Structure Exe-1 Goyal Brother Solutions ICSE Class-9 Ch-4
Questions 15. What do you understand by the following terms?
(a) Nucleus — The nucleus is the center of an atom. Almost all of the mass in an atom is made up from the protons and neutrons in the nucleus
(b) Nucleons– Nucleons are the protons and neutrons in the nucleus of an atom
Questions 16.
(a) Who discovered nucleus within an atom?–
Ans:– Rutherford and the Discovery of the Atomic Nucleus. In 1909
(b) Briefly describe the experiment which led to the discovery of nucleus.
Ans: Rutherford’s gold foil experiment showed that the atom is mostly empty space with a tiny, dense, positively-charged nucleus. Based on these results, Rutherford proposed the nuclear model of the atom
Questions 17. State two conclusions from Rutherford’s scattering of alpha particles with gold foil.
Answer : Almost 99% of the α-particles pass through the gold foil without any deflection. So atom must be having a lot of empty space in it. ii) Several α-particles get deflected at angle
Questions 18. Fill in the blanks spaces with appropriate words
(a) Almost all the mass of an atom concentrated in a small space within the atom, is called …Nucleus…
(b) The central part of an atom holding …Positive… charged parities and neutral …Neutrons… called nucleus.
(c) The volume of nucleus is …01% .. as compared to the volume of complete atom.
(d) The nucleus is positively charged, because of the presence of …Proton….
Questions 19. State Rutherford’s structure of atom.
Answer : The atom, as described by Ernest Rutherford, has a tiny, massive core called the nucleus. The nucleus has a positive charge. Electrons are particles with a negative charge. Electrons orbit the nucleus.
Questions 20. State the main drawback n Rutherford’s structure of atom.
Answer : Rutherford’s atomic model failed to explain the stability of electrons in a circular path. He stated that electrons revolve around the nucleus in a circular path, but particles in motion would undergo acceleration and cause energy radiation.
Questions 21. State Bohr’s model of the structure of atom.
Answer : According to Bohr Atomic model, a small positively charged nucleus is surrounded by revolving negatively charged electrons in fixed orbits. He concluded that electron will have more energy if it is located away from the nucleus whereas the electrons will have less energy if it located near the nucleus
Questions 22. What are shells around the nucleus? K,L M, N, O, P are the shells around the nucleus of an atom which shell is (a) nearest to nucleus and (b) farthest from nucleus?
Answer : The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus.
(a) nearest to nucleus called bound shell (b) farthest from nucleus called valence shell
Questions 23. What are neutrons? What experimental evidence led us to assume the existence of neutrons
Answer : A neutron is a subatomic particle found in the nucleus of every atom except that of simple hydrogen. The particle derives its name from the fact that it has no electrical charge; it is neutral. Neutrons are extremely dense.
Questions 24. Who discovered neutrons?
Answer : James Chadwick announced that the core also contained a new uncharged particle, which he called the neutron
Questions 25. Name three most important subatomic particles present in the nucleus of an atom.
Answer : The main three subatomic particles are Protons, electrons and neutrons
Questions 26. Fill in the blank spaces with appropriate words:
(a) Atoms have a central core called…Nucleus.. which is surrounded by ..Electron….
(b) The magnitude of ..positive.. charge on nucleus, is different for different …Elements..
(c) An atom is electrically ..Neutral.. Such that the total ….Positive…… charge on the nucleus, is equal to total …Negative… charge of electrons.
(d) The electrons have practically .negligible..mass as compared to the mass of nucleus.
(e) The charge on an electron is .. 6 × 10–19 .. Coulombs, which cannot be subdivided.
(f) All protons are identical and have a charge equal to …1.60 ×10–19.. coulombs.
(g) Neutrons have a mass equal to …Proton… but have ..No… electric charge.
(h) The volume of an atom is provided in some way by its …………..
Questions 27. What do you understand by the following terms:
(a) Atomic number –the number of protons that a chemical element has in its centre (nucleus)
(b) Mass number–The complete amount in the protons and neutrons in the nucleus of an atom.
Questions 28. An atom has mass number A and atomic number Z:
(a) How many protons are present in the nucleus?
(b) How many electrons are revolving around the nucleus?
(c) How many neutrons are present in its nucleus?
Answer :
(a) Z protons are present in the nucleus
(b) Z protons are present in the nucleus
(c) A-Z neutrons are present in its nucleus
Questions 29. An atom of an element is represented as 23X11.
(a) What does the numeral 23 indicate ?–
Ans:– Mass number
(b) What does the numeral 11 indicate ?–
Ans:- Atomic Number
(c) What is the number of protons in X ?
Ans —11
(d) What is the number of electrons in X ?
Ans —11
(e) How are the electrons distributed around the nucleus of X ?
Ans —11
(f) What is the number of neutrons in X? Neutron=12
Questions 30. Fill in the blank spaces with appropriate words:
(a) The number of protons in the nucleus of an atom is called its ..Atomic Number..
(b) The number of protons and neutrons present in the nucleus of an atom is called its ..Mass Number..
(c) An atom has the mass number 35 and the atomic number 17. The number of neutrons in its nucleus are …….35-17= 18
(d) An atom has 12 protons, 12 electrons and 12 neutrons, its mass number is ..24….. and atomic number is ..12…..
(e) An atom has mass number 32 and 16 neutrons. The number of electrons revolving around its nucleus are …..16…..
Questions 31. What do you understand by the term electronic configuration? State Bohr-Bury’s scheme of electronic configuration.
Answer : Electronic configuration is the arrangement of electrons around the nucleus of a particular atom or molecule. An atom is made of tiny particles called protons, neutrons, and electrons. A neutral atom has the same number of protons and elections
The rule of the Bohr-Bury Scheme can be stated as follows: -The electrons in the atom are arranged around the nucleus in different shells and the electrons first occupy that shell which is closest to the nucleus as it has the lowest energy. -The second shell is called the L-shell which can take upto eight electrons.
Questions 32. Calculate the number of neutrons in the following elements:
(a) 3H1
(b) 37Cl17
(c) 40Ar20 and
(d) 235H92
Answer :
We know that Neutron= Mass – Atomic Number so
(a) 3-1=2
(b) 37-17= 20
(c) 40-20 = 20
(d) 235-92=143
Questions 35. Write the electronic configuration of the following elements :
(a) 37Cl17
(b) 12C6
(c) 39K19
(d) 31P15
Answer :
(a) 2,8,8,7
(b) 2,4
(c) 2,8,8,1
(d) 2,8,5
Questions 34. Draw the atomic structure of the following elements geometrically, clearly showing the (a) number of protons (b) number of neutrons and (c) number of electrons in various shells around nucleus :
(a) 2H1
(b) 7Li3
(c) 27Al13
(d) 40Ca20
Answer :
(a) 
(b)
(c)
(d)
— End of Atomic Structure Exe-1 Goyal Brother ICSE Class-9 Ch-4. :–
Return to: ICSE Class-9 Chemistry Goyal Brothers Prakashan Solutions
Thanks



Man your most of the answers are wrong thats why I failed in exam i think that you should give answers according to textbook not by yourself
PLEASE 😶
ALL THE ANSWERS ARE WRONG OBJECTIVE AND SUBJECTIVE I SCORE LESS IN CHEMISTRY AND PHYSICS
I HAVE TO VERIFY ALL THE ANS FROM GOOGLE