Electric Charge and Fields Numericals on Quantization Class-12 Physics ISC Nootan Solutions Ch-1. Step by step solutions of Kumar and Mittal Physics of Nageen Prakashan as council latest prescribe guideline for upcoming exam. Visit official Website CISCE for detail information about ISC Board Class-12 Physics.
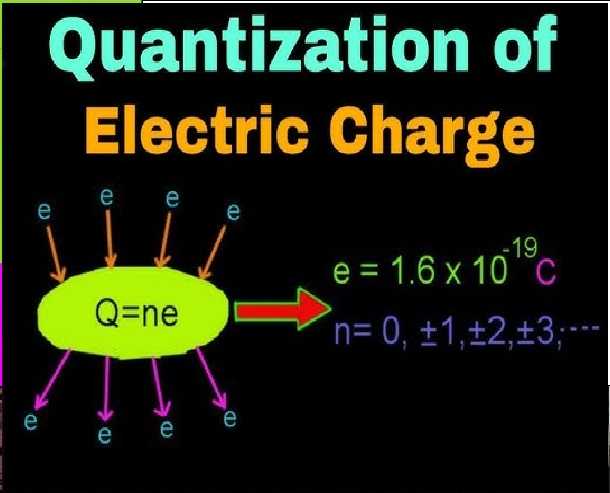
Electric Charge and Fields Numericals on Quantization Class-12 Physics ISC Nootan Solutions Ch-1
| Board | ISC |
| Class | 12 |
| Subject | Physics |
| Book | Nootan |
| Chapter-1 | Electric Charge and Fields Numerical-I ISC Class-12 Nootan ISC Physics Solutions Ch-1 |
| Topics | Numericals on Electric Charge and its Quantization |
| Academic Session | 2025-2026 |
Numericals on Electric Charge and its Quantization
Electric Charge and Fields Numerical-I ISC Class-12 Nootan ISC Physics Solutions Ch-1
Que-1: Calculate the number of electrons to have a total negative charge of 1 C
Ans: According to the quantisation of charge q = ne Here q = 1 C .
So the number of electrons in 1 coulomb of charge is
n = q/e
= 1C/(1.6×10^−19)
= 6.25×10^18 electrons.
Que-2: 6.25 x 10^12 electrons are removed from a glass rod. (i) What is the magnitude and nature of charge acquired by the glass rod? (ii) What will be the change in the mass of the rod? (mass of electron = 9.0 × 10^-31 kg)
Ans-2: (i) The charge of a single electron is −1.6×10^−19 coulombs.
Given that 6.25×10^12 electrons are removed from the glass rod, the total charge removed is:
Q = (6.25×10^12)×(1.6×10^−19 C)
Q = 10×10^−7C
Q = 1×10^−6 C
Q = 1 μC
(ii) The mass of a single electron is 9.0×10^−31 kg
Given that 6.25×10^12 electrons are removed, the total mass removed is:
Δm = (6.25×10^12)×(9.0×10^−31 kg)
Δm = 56.25×10^−19 kg
Δm = 5.625×10^−18 kg.
Que-3: Is the law of conservation of charge is valid in the following reaction?
20Ca44 + 1H1 → 21Sc44 + 0n1
Ans-3: To determine if the law of conservation of charge is valid in the given reaction, we need to check if the total charge before and after the reaction is conserved. The law of conservation of charge states that the total electric charge in an isolated system remains constant.
The given reaction is: 20Ca44 + 1H1 → 21Sc44 + 0n1
Reactants : Calcium has 20 protons, so the charge is +20.
Hydrogen has 1 proton, so the charge is +1.
Total charge of reactants: +20+1 = +21
Products : Scandium has 21 protons, so the charge is +21.
Neutron has 0 charge.
Total charge of products: +21+0 = +21
Since the total charge on the reactants side (+21) is equal to the total charge on the products side (+21), the law of conservation of charge is valid in this reaction.
— : End Electric Charge and Fields Numericals on Quantization Class-12 Physics ISC Nootan Solutions Ch-1 Questions :–
Return to : – Nootan Solutions for ISC Class-12 Physics
Thanks
Please share with your friends


