Gas Laws and Gas Equation Numerical Class-11 Nootan ISC Physics Ch-22 Behavior of Perfect Gas and Kinetic Theory of Gases. Step by step solutions of Kumar and Mittal Physics of Nageen Prakashan as council latest prescribe guideline for upcoming exam. Visit official Website CISCE for detail information about ISC Board Class-11 Physics.
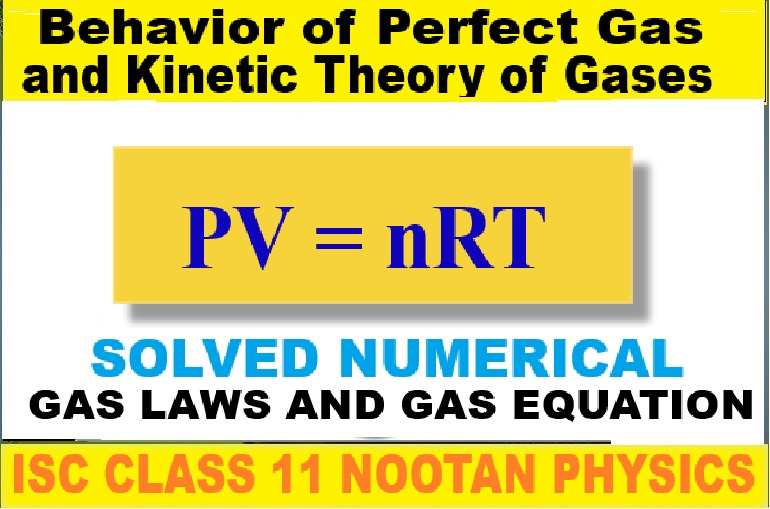
Gas Laws and Gas Equation Numerical Class-11 Nootan ISC Physics Ch-22 Behavior of Perfect Gas and Kinetic
| Board | ISC |
| Class | 11 |
| Subject | Physics |
| Book | Nootan |
| Chapter-22 | Behavior of Perfect Gas and Kinetic Theory of Gases |
| Topics | Numericals on Gas Laws and Gas Equation |
| Academic Session | 2025-2026 |
Numericals on Gas Laws and Gas Equation
Class-11 Nootan ISC Physics Ch-22 Behavior of Perfect Gas and Kinetic Theory of Gases
Que-1: The reading of a simple barometer containing some air above the mercury column is 73 cm, while the reading of a correct barometer is 76 cm. If the tube of the faulty barometer is pushed down into mercury until the volume of air in it is reduced to half its previous volume, what will be the new reading of the faulty barometer ?
Ans-1 Air pressure inside barometer = 76 – 73 = 3 cm
Let volume is V
when pressed in mercury volume = 1 / 2
According to Boyle’s Law
=> P1 V1 = P2 V2
=> 3 x V = P2 x V / 2
=> P2 = 6 cm
∴ new reading of barometer = 76 – 6 = 70 cm
Que-2: The volume of an air bubble becomes 11 times on reaching the surface of water from the bottom of a lake. If the height of mercury in the barometer be 75 cm, then calculate the depth of the lake. Density of water of the lake is 1.02 × 10³ kg m^-3.
Ans-2 Let the pressure due to water is Pw
now P1 V1 = P2 V2
=> (Pw + Po) V = Po 11 V
=> Pw = 11 Po – Po = 10 Po
now height of water column (depth of lake)
=> (0.75 x 13.6 x 10^3 x g) / (1.02 x 1 x 10^3 x g) = 100 m
Que-3: The pressure of an ideal gas filled in the bulb of a constant-volume gas thermometer at 7°C is 60 cm of mercury. What will be the pressure of the same volume of gas at 147°C?
Ans-3 P1 / P2 = T1 / T2
=> 60 / P2 = (273 + 7) / (273 + 147)
=> 60 / P2 = 280 / 420
=> P2 = 90 cm
Que-4; Air is filled in a bottle at atmospheric pressure and it is corked at 35°C. If the cork can come out at 3 atmospheric pressure then up to what temperature should the bottle be heated in order to remove the cork?
Ans-4 P1 / P2 = T1 / T2
=> P1 / 3 P1 = (273 + 35) / (273 + t)
=> 273 + t = 3 x 308 = 924
=> t = 924 – 273 = 651 °C
Que-5: The volume of a balloon filled partially with helium is 30 m³ at the earth’s surface where the pressure is 76 cm (mercury) and the temperature is 27°C. If this balloon rises up to a height where the pressure is 7.6 cm (mercury) and the temperature is – 54°C, then what will be the volume of the gas there?
Ans-5 P1 V1 / T1 = P2 V2 / T2
=> (76 x 30) / (273 + 27) = (7.6 x V2) / (273 – 54)
=> (76 x 30) / 300 = (7.6 x V2) / 213
=> V2 = (76 x 30 x 219) / (300 x 7.6)
=> V2 = 219 m³
Que-6: There are 4 x 10^24 gas molecules in a vessel at 50 K temperature. The pressure of the gas in the vessel is 0.03 atmospheric. Calculate the volume of the vessel.
Ans-6 P V = n K T
=> v = n K T / P
=> (4 x 10^24 x 1.38 x 10^-23 x 50) / (0.03 x 1.01 x 10^5) = 0.91 m³
Que-7: Calculate the number of molecules, volume occupied by one molecule and the average distance between two molecules in the 1.00 cm³ volume of gas at N.T.P.
Ans-7 n = P V / K T
=> (1.01 x 10^5 x 1 x 10^-6) / (1.38 x 10^-23 x 273) = 2.68 x 10^19
Volume occupied by one molecule
=> (1 x 10^-6) / (2.68 x 10^19) = 37.3 x 10^-27 m³
and average distance
=> (37.3 x 10^-27)^1/3 = 3.34 x 10^-9 m
Que-8: Calculate the value of Boltzmann constant k. Given : R = 8.3 x 10³ J (k mol)^-1 K^-1 and Avogadro number N = 6.03 × 10^26 (k mol)^-1.
Ans-8 K = R / N
=> (8.3 x 10^3) / (6.03 x 10^26) = 1.376 x 10^-23 J K^-1
Que-9: The volume of a gas at pressure 1.2 × 10^7 N m^-2 and temperature 127°C is 2.0 litre. Find the number of molecules in the gas.
Ans-9 n = P V / K T
=> (1.2 x 10^7 x 2 x 10^-3) / (1.38 x 10^-23 x (273 + 127)) = 4.35 x 10^24
Que-10: The residual pressure in a vessel, which is evacuated at 27°C, is 10^-11 N m^-2. Find the number of molecules per cm³ still remaining in the vessel.
Ans-10 n = P V / K T
=> (10^-11 x 1 x 10^-6) / (1.38 x 10^-23 x (273 + 27)) = 2415
Que-11: An electric bulb of volume 250 cm³ has been sealed at a pressure of 10^-3 mm of mercury and temperature 27°C. Find the number of air molecules in the bulb. What is the average distance between the molecules?
Ans-11 n = P V / K T
=> (250 x 10^-6 x 10^-3) / (1.38 x 10^-23 x (273 + 27)) = 8.0 x 10^15
volume occupied by each molecule
=> (250 x 10^-6) / (8 x 10^15) = 31.25 x 10^-21 m^3
∴ distance between molecules
=> (31.25 x 10^-21)^1/3 = 3.15 x 10^-7 m
Que-12: The temperature of a gas filled in a vessel is 273 K and the pressure is 1.60 x 10^-3 N m^-2. Determine:
(i) number of molecules in unit volume of the vessel,
(ii) average distance between the molecules.
Ans-12 (i) n = P V / K T
=> (1.6 x 10^-3 x 1) / (1.38 x 10^-23 x 273) = 4.25 x 10^17
=> (ii) average distance
=> (1 / 4.25 x 10^17)^1/3 = 1.33 x 10^-6 m
— : End of Gas Laws and Gas Equation Numerical Class-11 Nootan ISC Physics Ch-22. :–
Return to : – – Nootan Solutions for ISC Physics Class-11
Thanks
Please share with your friends


