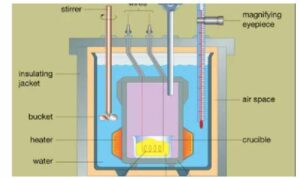
MCQ Calorimetry Class-10 ICSE for Sem-2 Sec-A . These MCQ / Objective Type Questions is based on latest reduced syllabus according 2021-22 session on bifurcated pattern. Main motto of MCQ Type Question is cracking the next upcoming Sem-2 exam of council. Visit official website CISCE for detail information about ICSE Board Class-10 Physical Education.
Sem-2 Sec-A MCQ Calorimetry Class-10 ICSE
| Board | ICSE |
| Class | 10th (X) |
| Subject | Physics |
| Chapter | Calorimetry |
| Syllabus | on bifurcated syllabus (after reduction) |
| Session | 2021-22 |
| Bifurcated | Sem-2 |
| Topic | MCQ / Objective Type Question for Sec-A |
ICSE Sem-2 Sec-A MCQ Calorimetry for Class-10
Question 1: The total internal energy of all molecules of a substance is called
(a) Temperature
(b) Heat energy
(c) Latent heat
(d) None of these
Answer: (b) Heat energy
Question 2: Between a cold and a hot body which body has higher energy?
(a) Hot body
(b) Cold body
(c) Both have same internal energy
(d) Cannot predict anything
Answer: (a) Hot body
Question 3: What will be happen when a cold body and hot body keeps in contact?
(a) Heat will flow from hot body to cold body
(b) Heat will flow from cold body to Hot body
(c) No heat exchanges
(d) None of these
Answer: (a) Heat will flow from hot body to cold body
Question 4:
(i)The measurement of heat is called calorimetry.
(ii) degree of hotness or coldness is calorimetry
(a) the option (i) is true but (ii) is false
(b) the option (ii) is true but (i) is false
(c) both true
(d) both false
Answer- (a) the option (i) is true but (ii) is false
Question 5: What is the S.I unit of heat?
(a) Calorie
(b) Kelvin
(c) Fahrenheit
(d) Joule
Answer: (d) Joule
Question 6: What is the C.G.S. unit of heat?
(a) Calorie
(b) Kelvin
(c) Fahrenheit
(d) Joule
Answer: (a) Calorie
Question 7: Find the required heat energy to raise the temperature of 1 g of water through 1?
(a) Joule
(b) Kelvin
(c) Fahrenheit
(d) Calorie
Answer: (d) Calorie
Question 8: One calorie is the heat energy required to raise the temperature of 1 g of water from ______ to _____
(a) 12.5 5 to 13.5
(b) 4 to 5
(c) 14.5 to 15.5
(d) 3 to 4
Answer: (c) 14.5 to 15.5
Question 9: 1 calorie = _________ joule
(a) 4.2
(b) 4.1
(c) 4.0
(d) None of these
Answer: (a) 4.2
Question 10: Which determines the direction of flow of heat?
(a) Temperature
(b) Latent heat
(c) Internal energy
(d) None of these
Answer: (a) Temperature
Question 11: Consider you contact two bodies (A, B) but after that you observe that there is no flow of heat between two bodies. From this incident you can say that
(a) The temperature Body A is higher than B
(b) The temperature Body B is higher than A
(c) Two bodies have same temperature
(d) You cannot say anything about the temperature
Answer: (c) Two bodies have same temperature
Question 12: The amount of heat energy contained in a body depends on
(a) Mass
(b) Temperature
(c) Nature of the body
(d) All of above
Answer-(d) All of above
Question 13: What is the S.I unit of Temperature?
(a) Degree Celsius
(b) Kelvin
(c) Fahrenheit
(d) Joule
Answer: (b) Kelvin
Question 15: What is the relation between the kelvin (T k) scale and degree Celsius is
(a) K+273 =T
(b) T+273 = K
(c) T-273 =K
(d) None of above
Answer-(b) T+273 = K
Question 16: At which temperature molecular motion ceases?
(a) -273 K
(b) 273 K
(c) 0 K
(c) 0 C
Answer: (c) 0 K
Question 17: What is the relation between amount of heat energy absorbed by a body and rise in temperature?
(a) They are directly proportional to each other
(b) They are inversely proportional to each other
(c) There is no relation between them
(d) None of these
Answer: (a) They are directly proportional to each other
Question 18: The_____________ of a body is the amount of heat energy required to raise its temperature by 1 k
(a) Heat
(b) Temperature
(c) Heat capacity
(d) Latent heat
Answer: (c) Heat capacity
Question 19 : What is the unit of heat capacity?
(a) J / K
(b) J / C
(c) Both
(d) None of the above
Answer- (a) J / K
Question 24: Heat capacity per unit mass of a body is called
(a) Latent heat
(b) Specific heat
(c) Heat energy
(d) None of these
Answer: (b) Specific heat
Question 25: What is the S.I unit of specific heat capacity?
(a) J / Kg K
(b) J /Kg / K
(c) J / Kg C
(d) J C / Kg
Answer- (a) J / Kg K
Question 26: What is the relation between specific heat capacity(C’) and heat capacity (c) of a body of mass m?
(a) C’ =c/m
(b) C’ =m× c
(c) c= C’/m
(d) c=m× C’
Answer: (a) C’=c/m
Question 28: The specific heat capacity of water is
(a) 420
(b) 4200
(c) 4.2
(d) 42000
Answer: (b) 4200
Question 29: Heat is measured by
(a) Calorimeter
(b) Thermometer
(c) Lactometer
(d) Hydrometer
Answer: (a) Calorimeter
Question 30 Latent heat of vaporization is
(a)the amount of heat required to raise the temperature of a 1 kg of a substance by 1 K
(b)the amount of heat required to raise the temperature of a substance by 1 K
(c) the amount of heat required to change the phase of a substance from solid to liquid without any change in temperature
(d) the amount of heat required to change the phase of a substance from liquid to gas without any change in temperature
Answer: (d) the amount of heat required to change the phase of a substance from liquid to gas without any change in temperature
Question 31 Latent heat of fusion of ice is
(a)4.36 × 105 Jkg-1
(b)2.36 × 105 Jkg-1
(c)1.36 × 105 Jkg-1
(d)3.36 × 105 Jkg-1
Answer: (d)3.36 × 105 Jkg-1
–: End of MCQ Calorimetry Class-10 ICSE :–
-: also visit :-
Please share with your ICSE friends if it is helpful
Thanks