Numericals on Work Done in Isothermal and Adiabatic Processes Class-11 Nootan ISC Physics Solutions Ch-20. Step by step solutions of Kumar and Mittal Physics of Nageen Prakashan as council latest prescribe guideline for upcoming exam. Visit official Website CISCE for detail information about ISC Board Class-11 Physics.
Numericals on Work Done in Isothermal and Adiabatic Processes Class-11
(Nootan ISC Physics Solutions Ch-20)
| Board | ISC |
| Class | 11 |
| Subject | Physics |
| Book | Nootan |
| Chapter-20 | Isothermal and Adiabatic Processes |
| Topics | Numericals on Work Done in Isothermal and Adiabatic Processes |
| Academic Session | 2024-2025 |
Numericals on Work Done in Isothermal and Adiabatic Processes
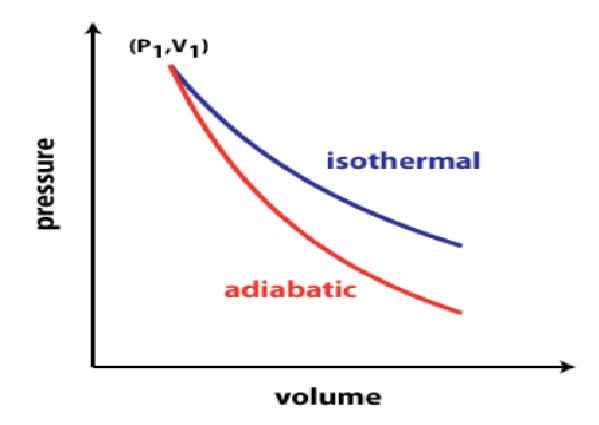
The work done in an isothermal process is due to the change in the net heat content of the system. Meanwhile, the work done in an adiabatic process is due to the change in its internal energy
page-780
Que-11: Two moles of oxygen at 0°C are compressed until the volume remains one-fourth of the initial value at the same temperature. Calculate the work done. R = 8.31 J / mol-K.
Ans: work done during isothermal process
=> W = 2.3.26 RT log V2/V1
=> 2.3026 x 8.31 x 273 x log (V1/4 V1)
=> 2.3026 x 8.31 x 273 x log 2^-2
=> -2.3026 x 8.31 x 273 x 2 x 0.3010
=> – 6290 J
Que-12: An ideal gas of volume 1 liter and at pressure 8 atmospheres expands adiabatically until the pressure drops to 1 atmosphere. Find the final volume and work done. Given : γ = 1.5, 1 atmosphere = 1.013 × 10^5 N/m² and 1 litre = 10^-3 m³.
Ans: For adiabatic process
=> P1 V1^γ = P2 V2^γ
=> P1/P2 = (V2/V1)^γ
=> (8/1) = (V2/1)^γ
=> V2 = 8^(1/γ)
=> V2 = 8^(2/3)
=> V2 = 4 Litre
again work done
=> (1 / γ-1) (P1 V1 – P2 V2)
=> [1 / (1.5 – 1)] (10^-3 x 8 x 1.013 x 10^5 x -4 x 10^-3 x 1.013 x 10^5)
=> 810.4 J
Que-13: One mole of oxygen at NTP is compressed adiabatically to 5 atmospheres. What is the new temperature and the work done? Given: γ = 1.4 and R = 8.31 J / mol-K.
Ans: For adiabatic process
=> (T1 / T2) = (P1 / P2)^(γ-1 / γ)
=> 273 / T2 = (P1 / 5 P1)^(1.4-1 / 1.4)
=> T2 = 432 K
=> 432 – 273 = 159 °C
again work done
=> (R / γ-1) (T1 – T2)
=> (8.31 / 1.4 – 1) (273 – 432)
=> – 3299 J
— : end of Numericals on Work Done in Isothermal and Adiabatic Processes Class-11 Nootan ISC Physics Solutions :–
Return to : – Nootan Solutions for ISC Class-11 Physics
Thanks
Please Share with your friends if helpful