Study of First Element Hydrogen Exe-6D Structured Answer Chemistry Class-9 ICSE Selina Publishers Solutions Chapter-6. Step By Step ICSE Selina Concise Solutions of Chapter-6 Study of First Element Hydrogen with All Exercise including MCQs, Very Short Answer Type, Short Answer Type, Long Answer Type, Numerical and Structured/Application Questions Solved . Visit official Website CISCE for detail information about ICSE Board Class-9.
Study of First Element Hydrogen Exe-6D Structured Answer Chemistry Class-9 ICSE Concise Selina Publishers
| Board | ICSE |
| Publications | Selina Publication |
| Subject | Chemistry |
| Class | 9th |
| Chapter-6 | Study of First Element Hydrogen |
| Book Name | Concise |
| Topics | Solution of Exercise – 6D Structured/Application/Skill Answer Type |
| Academic Session | 2023-2024 |
D. Exercise – 6D Structured/Application/Skill Answer Type
Study of First Element Hydrogen Class-9 Chemistry Concise Solutions
Page-119
Question 1.
Look at the following figure and answer the questions that follow:
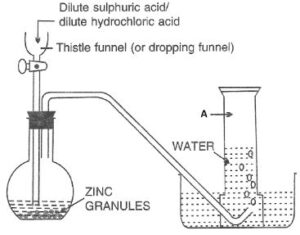
(a) Which gas is prepared by this method marked as A.
(b) Name this method of collection. Why is this method used?
(c) Why is nitric acid not used as a reactant in the above method?
(d) Conc. H2SO4 is a good drying agent. However, it is not used here. Why?
Answer:
(a) Hydrogen
(b) Downward displacement of water.
This method is used as:
- Hydrogen is virtually insoluble in water (20 ml of hydrogen dissolves in 1 litre of water under normal conditions)
- Hydrogen forms an explosive mixture with air and therefore, cannot be collected by downward displacement of air even though it is lighter than air.
(c) Nitric acid, even in its dilute form, is not used in the preparation of hydrogen from metals because it is a powerful oxidizing agent, and the oxygen formed due to its decomposition oxidizes the hydrogen to give water, thus defeating the purpose of the reaction.
3Zn + 8HNO3 ⟶ 3Zn(NO3)2 + 4H2O + 2NO↑
(d) Conc. H2SO4 is a good drying agent. However, it is not used to dry hydrogen as it reacts with hydrogen, thus defeating the purpose of the reaction.
H2SO4 + H2 ⟶ 2H2O + SO2
— : End of Study of First Element Hydrogen Exe-6D Structured Answer Class-9 ICSE Chemistry Solutions :–
Return to Return to Concise Selina ICSE Chemistry Class-9
Thanks
Please share with your friends


