Study of Gas Laws Exe-7 Descriptive Answer Chemistry Class-9 ICSE Selina Publishers Solutions Chapter-7. Step By Step ICSE Selina Concise Solutions of Chapter-7 Study of Gas Laws with All Exercise including MCQs, Very Short Answer Type, Short Answer Type, Long Answer Type, Numerical and Structured/Application Questions Solved . Visit official Website CISCE for detail information about ICSE Board Class-9.
Study of Gas Laws Exe-7 Descriptive Answer Chemistry Class-9 ICSE Concise Selina Publishers
| Board | ICSE |
| Publications | Selina Publication |
| Subject | Chemistry |
| Class | 9th |
| Chapter-7 | Study of Gas Laws |
| Book Name | Concise |
| Topics | Solution of Exercise – 7 Descriptive Answer Type |
| Academic Session | 2023-2024 |
D. Exercise – 7 Descriptive Answer Type
Study of Gas Laws Class-9 Chemistry Concise Solutions
Page-132
Question 1.
Give the assumptions of the kinetic molecular theory.
Answer:
- Gases are made of tiny particles which move in all possible directions at all possible speeds. The size of molecules is small as compared to the volume of the occupied gas.
- There is no force of attraction between gas particles or between the particles and the walls of the container. So, the particles are free to move in the entire space available to them.
- The moving particles of gas collide with each other and with the walls of the container. Because of these collisions, gas molecules exert pressure. Gases exert the same pressure in all directions.
- There is large inter particle space between gas molecules, and this accounts for high comprehensibility of gases.
- Volume of a gas increases with a decrease in pressure and increase in temperature.
- Gases have low density as they have large inter molecular spaces between their molecules.
- Gases have a natural tendency to mix with one and other because of large inter molecular spaces. So, two gases when mixed form a homogeneous gaseous mixture.
- The inter molecular space of a gas is reduced because of cooling. Molecules come closer resulting in liquefaction of the gas.
Question 2.
Explain Boyle’s Law on the basis of the kinetic theory of matter.
Answer:
Boyle’s law on the basis of the kinetic theory of matter:
- According to the kinetic theory of matter, the number of particles present in a given mass and the average kinetic energy is constant.
- If the volume of the given mass of a gas is reduced to half of its original volume, then the same number of particles will have half the space to move.
- As a result, the number of molecules striking the unit area of the walls of the container at a given time will double and the pressure will also double.
- Alternatively, if the volume of a given mass of a gas is doubled at constant temperature, the same number of molecules will have double the space to move.
- Thus, the number of molecules striking the unit area of the walls of a container at a given time will become one-half of the original value.
- Thus, pressure will also get reduced to half of the original pressure. Hence, it is seen that if the pressure increases, the volume of a gas decreases at constant temperature, which is Boyle’s law.
Question 3.
Explain Charles’s law on the basis of the kinetic theory of matter.
Answer:
Charles’s law on the basis of the kinetic theory of matter:
According to the kinetic theory of matter, the average kinetic energy of gas molecules is directly proportional to the absolute temperature. Thus, when the temperature of a gas is increased, the molecules would move faster and the molecules will strike the unit area of the walls of the container more frequently and vigorously. If the pressure is kept constant, the volume increases proportionately. Hence, at constant pressure, the volume of a given mass of a gas is directly proportional to the temperature (Charles’s law).
Question 4.
How did Charles’s law lead to the concept of absolute scale of temperature?
Answer:
- The temperature scale with its zero at -273°C and where each degree is equal to the degree on the Celsius scale is called the absolute scale of temperature.
- The temperature -273°C is called absolute zero. Theoretically, this is the lowest temperature which can never be reached. All molecular motion ceases at this temperature.
- The temperature -273°C is called absolute zero.
Question 5.
(a) State Boyle’s Law.
(b) Give its
(i) Mathematical expression
(ii) Graphical representation and
(iii) Significance
Answer:
(a) Boyle’s law:
At constant temperature, the volume of a definite mass of any gas is inversely proportional to the pressure of the gas.
Or
Temperature remaining constant, the product of the volume and pressure of the given mass of a dry gas is constant
(a) Mathematical representation:
(i)
iii. Significance of Boyle’s law:
According to Boyle’s law, on increasing pressure, volume decreases. The gas becomes denser. Thus, at constant temperature, the density of a gas is directly proportional to the pressure.
At higher altitude, atmospheric pressure is low so air is less dense. As a result, lesser oxygen is available for breathing. This is the reason mountaineers carry oxygen cylinders.
Question 6.
(a) State Charles’s law.
(b) Give its
(i)Graphical representation
(ii) Mathematical expression and
(iii) . Significance
Answer:
(a) Charles’s Law
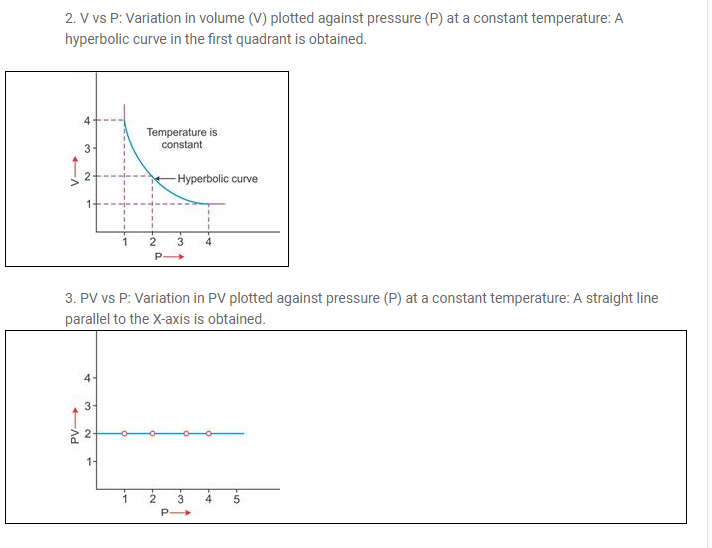
At constant pressure, the volume of a given mass of a dry gas increases or decreases by 1/273rd of its original volume at 0°C for each degree centigrade rise or fall in temperature.
| V ∝ T (at constant pressure) |
At temperature T1 (K) and volume V1 (cm3):
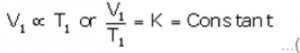 …(i)
…(i)
At temperature T2 (K) and volume V2 (cm3):
 ….(ii)
….(ii)
From (i) and (ii),
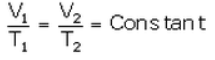
For Temperature = Conversion from Celsius to Kelvin
1 K = °C + 273
Example:
20°C = 20 + 273 = 293 K
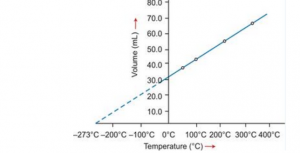
(b) Graphical representation of Charles’s law
T vs V: The relationship between the volume and the temperature of a gas can be plotted on a graph. A straight line is obtained.
|
Graphical representation of Charles’s law |
Significance of Charles’s Law: The volume of a given mass of a gas is directly proportional to its temperature; hence, the density decreases with temperature. This is the reason that
(a) Hot air is filled in balloons used for meteorological purposes. (b) Cable wires contract in winters and expand in summers.
Question 7.
What is meant by aqueous tension? How is the pressure exerted by a gas corrected to account for aqueous tension?
Answer:
Gases such as nitrogen and hydrogen are collected over water as shown in the diagram. When the gas is collected over water, the gas is moist and contains water vapour. The total pressure exerted by this moist gas is equal to the sum of the partial pressures of the dry gas and the pressure exerted by water vapour. The partial pressure of water vapour is also known as aqueous tension.
| Collection of gas over water |
Ptotal = Pgas + Pwater vapour
Pgas = Ptotal– Pwater vapour
Actual pressure of gas = Total pressure – Aqueous tension
Question 8.
During the practical in the lab when hydrogen sulphide gas having offensive odour is prepared for some test, we can smell the gas even 50 metres away. Explain the phenomenon.
Answer:
The phenomenon is Diffusion.
Diffusion is the process of gradual mixing of two substances, kept in contact, by molecular motion.
The molecules of hydrogen sulphide gas collide with air particles and due to the collisions of the particles, they start moving in a haphazard manner in all possible directions. Due to this, the molecules of hydrogen sulphide gas quickly spread in the surroundings and we can smell the gas from even 50 metres away.
— : End of Study of Gas Laws Exe-7 Descriptive Answer Class-9 ICSE Chemistry Solutions :–
Return to Return to Concise Selina ICSE Chemistry Class-9
Thanks
Please share with your friends