Sulphuric Acid Dalal Simplified ICSE Chemistry Class-10 Solutions. Dalal Simplified ICSE Chemistry Sulphuric Acid by Dr Viraf and J Dalal for Class 10. Step by step Solutions of Sulphuric Acid by Dr Viraf and J Dalal of Concept of Simplified Dalal ICSE Chemistry.
Sulphuric Acid Dalal Simplified ICSE Chemistry Class-10
Get Other Chapter Dalal Simplified ICSE Chemistry Class-10 Solutions
How to Solve Mole Concept ICSE Chemistry Class-10
Note:– Before viewing Solutions of
How to Solve Mole Concept ICSE Chemistry Class-10
Note:– Before viewing Solutions of Sulphuric Acid by Dr Viraf and J Dalal Simplified ICSE Chemistry Solutions .Read the Sulphuric Acid And Stoichiometry Carefully to understand the concept in better way .After reading the Sulphuric Acid solve all example of your text book with ICSE Specimen Sample Paper for Class-10 Exam of Council. Focus on Numerical’s Problems. Mole Concept is the Most important Chapter in ICSE Class 10 Chemistry.Previous Year Solved Question Paper for ICSE Board
ADDITIONAL QUESTIONS Sulphuric Acid Dalal Simplified ICSE
Question 1.
State why sulphuric acid was called – ‘oil of vitriol’.
Answer:
Sulphuric acid was initially called ‘oil of vitriol ’.It was initially prepared by – distilling green vitriol [FeSO4.7H2O] and hence the name – ‘oil of vitriol’.
Question 2.
State how you would convert
(1) sulphur
(2) chlorine
(3) sulphur dioxide – to sulphur acid.
Answer:
- S + 6HNO. [cone.] → 6NO2 + 2H2O + H2SO4
- Cl2 + SO2 + 2H2O → 2HCl + H2SO4
- 3SO2 + 2HNO3 + 2H2O → 3H2SO4 + 2NO
Question 3.
State the purpose of the ‘Contact process’.
Answer:
When sulphur is burnt in air, it bums with a pale blue flame forming sulphur dioxide and traces of sulphur trioxide.
S + O2→ SO2 [2S + 3O2 → 2SO4 (traces)]
Burning of sulphur or iron pyrites in oxygen is preferred to purified air since heat energy is wasted in heating the unreactive nitrogen component of the air.
Question 4.
In the Contact process
- State how you would convert (a) sulphur (b) iron pyrites to sulphur dioxide in the first step of the Contact process.
- State the conditions i.e. catalyst, promoter, temperature and pressure in the catalytic oxidation of sulphur dioxide to sulphur trioxide in the Contact tower. Give a balanced equation for the same.
- State why the above catalytic oxidation {reaction supplies energy.
- Give a reason why – vanadium pentoxide is preferred to platinum during the catalytic oxidation of sulphur dioxide.
- Give a reason why the catalyst mass is heated electrically – only initially.
- State why sulphur trioxide vapours are absorbed in concentrated sulphuric acid and not in water to obtain sulphuric acid.
Answer:
1 S + O2 → 5- SO2
4FeS2 + 11O2 → 5- 2Fe2O3 + 8SO2
2 Catalytic oxidation of sulphur dioxide to sulphur trioxide.
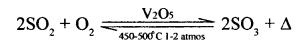
[Above equation for the catalysed reaction is exothermic- hence supplies energy.]
Catalyst: Vanadium pentoxide [V2O5] or platinum [Pt],
Temperature: 450-500°C Pressure : 1 to 2 atmospheres
Conversion ratio: 98% of sulphur dioxide converted to sulphur trioxide.
3 The catalytic oxidation of SO2 to SO3 is an – exothermic reaction. Thus this reaction supplies energy in the form of heat.
![]()
4 Vanadium pentoxide is preferred – to platinized asbestos as a catalyst since it is comparatively – cheaper and less easily poisoned or susceptible to impurities.
5 The catalyst – mass is – only initially heated electrically, since the catalytic oxidation of sulphur dioxide is an exothermic reaction and the heat produced maintains the temperature at 450 – 500°C.
6 Even though sulphur trioxide is an acid anhydride of sulphuric acid – it is not directly absorbed in water to give sulphuric acid.
The reaction is highly exothermic resulting in production of – a dense fog of sulphuric acid particles which do not condense easily.
Hence sulphur trioxide vapours are – dissolved in cone, sulphuric acid to give oleum which on dilution with – the requisite amount of soft water in the dilution tank gives – sulphuric acid of the desired concentration [about 98%].
Question 5.
Give a reason why concentrated sulphuric acid is kept in air tight bottles.
Answer:
Concentrated sulphuric acid has a great affinity for water and as such it is a hygroscopic liquid. Being Hygroscopic, it absorbs moisture from the atmosphere and hence cone, sulphuric acid is kept in air tight bottles.
Question 6.
State the basic steps with reasons, involved in diluting a beaker of cone. H2SO4.
Answer:
For dilution of cone. H2SO4, the cone, acid is always added to water and water is never added to the cone, acid even though heat is evolved in both cases.
Reason: If water is added to cone. H2SO4 the heat produced is sufficient to spontaneously vaporise a part of the few drops of water added. This is because the amount of water is very small and bioling point of water is much lower than cone. H2SO4, which is in bulk. Due to this sudden vaporisation of water cone, acid tend to spurt out and cause serious injuries.
On the other hand, if cone. H2SO4 is added to water, the heat produced in this case can only raise the temperature of water slightly because water is an bulk. Thus, in this case spurting of the cone, acid is avoided.
Question 7.
Give reasons why dilute sulphuric acid:
(1) behaves as an acid when dilute.
(2) is dibasic in nature.
Answer:
1. Acidic properties of sulphuric acid are due to the presence of – hydronium ions [H3O+ ] formed when H2SO4 dissociates in aq. solution
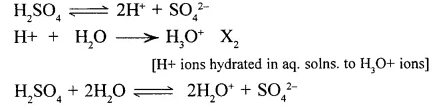
2. Sulphuric acid dissociates in – aq. solution giving 2H+ ions per molecule – of the acids .Hence its – basically is two.
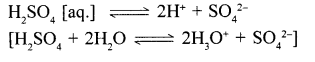
[Basicity is the number of H+ ions formed by dissociation of one molecule of the acid in its aq. soln.]
Question 8.
Convert dil. H.SO, to –
- Hydrogen
- Carbon dioxide
- Sulphur dioxide
- Hydrogen sulphide
- An acid salt
- A normal salt.
Answer:
1. Dil. H2S04to sulphur dioxide by the action of any sulphide on dil. H2SO4
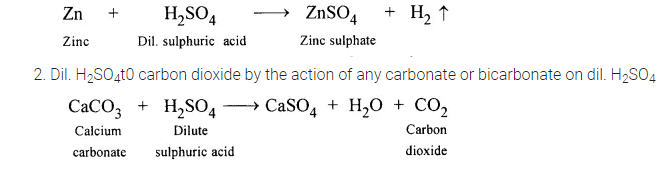
3. Dil. H2S04to sulphur dioxide by the action of any sulphide on dil. H2SO4
Na2SO3 + H2SO4 → Na2SO4 + H2O + SO2
4. H2SO4 to hydrogen sulphide by the action of any sulphide on dil. H2SO4
FeS + H2SO4 (dil.) → FeSO4 + H2S↑
5. H2S04to an acid by the action of insufficient strong base with excess of dil. H2SO4
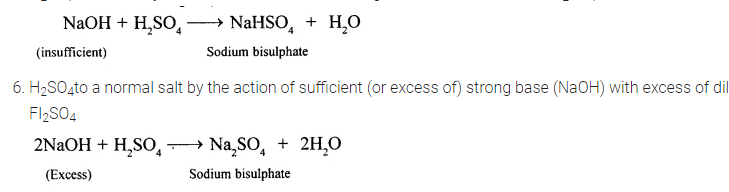
Question 9.
Give equations for formation of two different acids from cone. H2SO4. State the property of sulphuric acid involved in the above formation.
Answer:
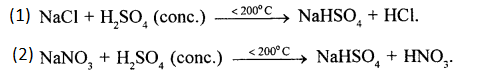
Property of Sulphuric acid involved in the formation of these acids: Cone. H2SO4 is a non-volatile acid.
Question 10.
Give equations for oxidation of cone. H2SO4 giving the oxidised products –
- Carbon dioxide
- Sulphur dioxide
- Phosphoric acid
- Copper (II) sulphate
- Iodine
- Sulphur respectively.
Answer:
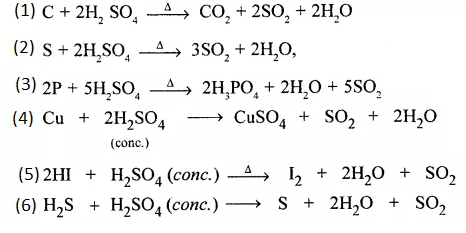
Question 11.
Give a reason why concentrated and not dil H2SO4 – behave as an oxidising and dehydrating agent.
Answer:
1. Cone. H2SO4 be aves as an oxidising agent because cone.H2SO4 whes h. ated decomposes to give nascent oxygen which acts as a strong oxidising agent.On the other hand, dil H2SO4 on heating does not decompose to give nascent oxygen and as such cannot behave as an oxidising agent.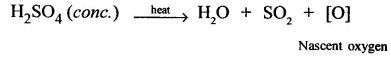
2. Cone. H2SO4 behaves as a dehydrating agent because cone. H2SO4 act as a great affinity for water and hydration of cone. H2SO4 is an exothermic reaction.On the other hand, dil H2SO4 has no affinity for water and hence cannot act as a hydrating agent.
Question 12.
Give the equation for the reaction cone, sulphuric acid with –
- glucose
- sucrose
- cellulose
- an organic acid containing one carbon atom and two hydrogen atoms
- an organic acid containing two carbon and two hydrogen atoms
- an alcohol
- hydrated copper (II) sulphate.
Answer:

Question 13.
State the observation seen when cone. H2SO4 is added to –
(1) sucrose
(2 ) hydrated copper (II) sulphate.
Answer:
1. Cone. H2SO4 dehydrates sucrose to carbon, called sugar charcoal. This is m the form of a black spongy charged mass of carbon.
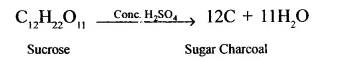
2. Cone. H2S04 dehydrates blue crystals of copper (II) sulphate pentahydrate (blue vitriol) to copper sulphite, which is in the form of a white powder.
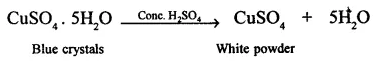
Question 14.
State how addition of –
- copper
- NaCl to hot cone. H2SO4 serves as a test for the latter.
Answer:
1.Copper turnings when heated with cone. H2SO4 gives a colourless suffocating gas with a smell of burning sulphur(SO2). The gas turns orange coloured acidified potassium dichromate solution green. This can be used as a test for cone. H2SO4.
![]()
2.Common salt (NaCl) when heated with cone. H2SO4 gives a colourless gas pungent smell only which (HCl) gives white fumes with NH3. This can be used as a test for cone. H2SO4.
![]()
Question 15.
Give two tests for dilute sulphuric acid with balanced equations. State why
(1) BaCl2
(2) Pb(NO3)2 are used for the above tests.
Answer:
- Barrium chloride solution on treating with dilute sulphuric acid forms white ppt. of barium sulphate, which is insoluble in all acids
BaCl2 + H2SO4 (dil) → 2HCl + BaS04 ↓ - Lead nitrate on treating with dilute sulphuric acid forms white ppt of lead sulphate, which is insoluble in all acids.
Pb (NO3)2 + H2SO4 (dil) → 2HNO3 + PbSO4 ↓
Question 16.
Give a test to distinguish dilute sulphuric acid from dilute HCl and dilute HNO3.
Answer:
Test to distinguish dil H2SO4 from dil HCl and HNO3- BaCl2 soln. when added to dil. H2S04 gives a white ppt. of BaSO4, but with dil. HCl and dil. HNO3, no white ppt. is produced – since BaCl2 and Ba(NO3)2 are soluble in dil. H2SO4.
Question 17.
State three different chemical compounds other than acids manufactured industrially from sulphuric acid.
Answer:
- Barium sulphate
- Lead sulphate
- Sodium sulphate
–Try Also :–