Variations of Properties in Periodic Table for Class 10 ICSE Chemistry The first chapter of ICSE Class 10 Chemistry is Periodic Table. In this article you would get notes and important point on Variations of Properties in Long Form of PT. Visit official website CISCE for detail information about ICSE Board Class-10 Chemistry.
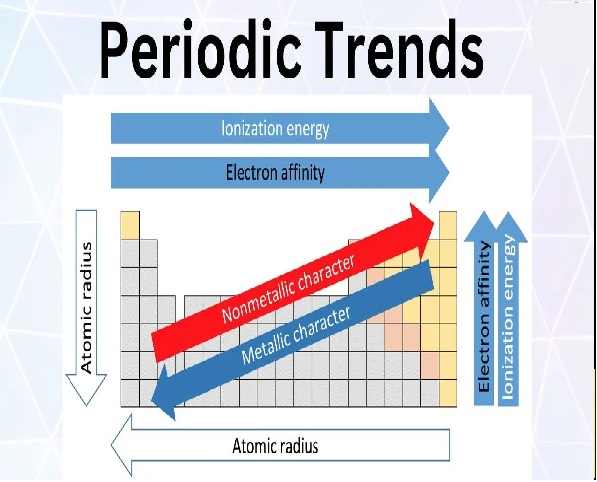
Variations of Properties in Periodic Table for Class 10 ICSE Chemistry
Point to be Discuss |
Atomic Radius |
Ionisation Potential |
Electron Affinity |
Electro-Negativity |
Non Metallic Character |
Metallic Character |
Atomic Radius / Size
the atomic radius is the distance between its nucleus and its outermost stable electron orbital.
Number of Shell Increase then Size Increase
Nuclear Charge Increase then size Decrease
Ionisation Potential
“Minimum energy required by an isolated atom to remove one electron in its neutral or gaseous state”
Number of Shell Increase then Size Decrease
Nuclear Charge Increase then size Increase
Electron Affinity
tendency of an atom to accept an electron or an electron pair.
Number of Shell Increase then Electron Affinity Decrease
Nuclear Charge Increase then Electron Increase
Electro-Negativity
Electronegativity is the ability of an atom or molecule to attract a pair of electrons
Number of Shell Increase then Electro-Negativity Decreases
Nuclear Charge Increase then Electro-Negativity Increases
Non Metallic Character
Number of Shell Increase then Non-metallic character Decreases
Nuclear Charge Increase then Non-metallic Increases
Metallic Character
Number of Shell Increase then Metallic character Increases
Nuclear Charge Increase then Metallic Decreases
— : End of Variations of Properties in Periodic Table for Class 10 ICSE Chemistry :–
- Concise Selina Chemistry Solutions for ICSE Class-10.
- Simplified Dr. Dalal Chemistry Solutions for ICSE Class-10
- Goyal Brothers Prakashan for ICSE Class-10 Chemistry Textbook
Thanks
Please Share with Your Friend