Water MCQs Class-8 Dalal Simplified ICSE Chemistry Solutions Chapter-8, Water Dr Viraf J Dalal Middle School Allied Publishers Solutions. Chapter-8. We Provide Step by Step Solutions to Correct Answer, Give balanced equation, Fill in the blanks, Give reasons, Match the following of Dr Viraf J Dalal Middle School Chemistry Allied Publishers. Visit official Website CISCE for detail information about ICSE Board Class-8.
Water MCQs Class-8 Dalal Simplified ICSE Chemistry Solutions Chapter-8
| Board | ICSE |
| Class | 8th |
| Subject | Chemistry |
| Book Name | Dalal New Simplified |
| Chapter-8 | Water |
| Unit-1 | Water |
| Topic | Solution of exercise MCQs |
| Session | 2023-24 |
Objective Types Questions
Water MCQs Class-8 Dalal Simplified ICSE Chemistry Solutions Chapter-8
Question: 1. Select the correct answer from A, B, C, D & E for each statement given below :
A : Colloidal B : Fused calcium chloride C : Solvent D : Suspension E : Washing soda
| 1. The medium of dissolution which allows the solute to dissolve in it. | C : Solvent |
| 2. A solution which can pass through a filter paper but not through a semipermeable membrane. | A : Colloidal |
| 3. A decahydrate crystal | E : Washing soda |
| 4. A drying agent placed in a desiccator | B : Fused calcium chloride |
| 5. A heterogeneous mixture of undissolved particles in dispersion medium, visible to the naked eye. | D : Suspension |
Question: 2. Give a balanced equation for the following conversions:
1. Calcium sulphate in permanent hard water to calcium carbonate using sodium carbonate.
2. Iron to tri-iron tetra-oxide using steam
3. Sulphur dioxide to sulphurous acid using a neutral liquid
4. Potassium oxide to a strong alkali.
5. Magnesium bicarbonate in temporary hard water to magnesium carbonate by boiling.
Answer:
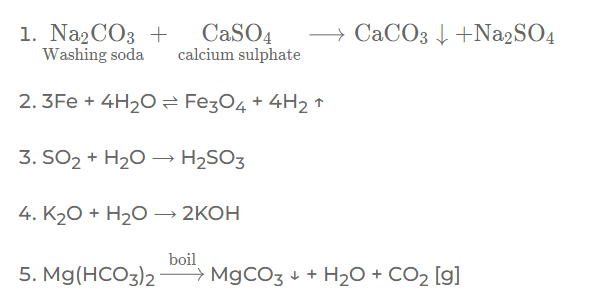
Question: 3. Complete the statements by filling the blanks with the correct word from the bracket:
1. Solubility of most solids __increases__ (decreases/increases)
2. Kerosene & water form a __immiscible__ (miscible/immiscible) mixture.
3. Solubility od a solute is the __maximum__ (minimum/maximum) amount of solute that will saturate 100g of water at 1^oC.
4. Hygroscopic substance absorbs moisture from the atmosphere & __do not change__(do not change/change) their original state.
5. The ratio of hydrogen & oxygen in water is __2:1__ (2:1/1:2)
Question: 4. Give reasons for the following:
1. All solutions are homogeneous mixtures of a solute in a solvent.
2. Hardness in temporary water can be removed by boiling but the hardness in permanent hard water cannot.
3. Colloidal solutions exhibit Brownian movement.
4. The percentage of oxygen, in the air dissolved in water, is higher than the percentage of oxygen in ordinary air.
5. Washing soda can be used to remove both temporary and permanent hardness in water.
Answer: 1. As solute dissolves in the solvent and forms a uniform composition throughout hence, all solutions are homogenous mixtures of a solute in a solvent.
2. On boiling, the calcium and magnesium bicarbonates responsible for temporary hardness in water, decompose into insoluble carbonates and carbon dioxide which are filtered out making water soft. The chlorides and sulphates of calcium and magnesium responsible for permanent hardness in water, are stable to heat hence, permanent hardness cannot be removed by boiling.
3. Colloidal solutions exhibit Brownian movement due to the constant bombardment of molecules of the dispersion medium on the colloidal particles.
4. Ordinary air contains 21% oxygen. As oxygen is more soluble in water as compared to air. Hence, air in water contains 33% oxygen which is more than that in ordinary air.
5. On adding washing soda to water containing calcium & magnesium chlorides, sulphates & bicarbonates an insoluble precipitate of calcium & magnesium carbonate is formed which is filtered out. The remaining soln. contains soluble sodium salts which do not cause hardness in water & hence the residual water is rendered soft. Hence, washing soda can be used to remove both temporary and permanent hardness in water.
Question: 5. Match the substances in List I with the appropriate answer in List II:
| List I | List II |
| 1. Green vitriol | A: Permanent hardness in water |
| 2. Paint | B: Hygroscopic |
| 3. Magnesium chloride | C: Temporary hardness in water |
| 4. Magnesium bicarbonate | D: Heptahydrate |
| 5. Calcium oxide | E: Colloidal |
Answer:
| List I | List II |
| 1. Green vitriol | A: Heptahydrate |
| 2. Paint | B: Colloidal |
| 3. Magnesium chloride | C: Permanent hardness in water |
| 4. Magnesium bicarbonate | D: Temporary hardness in water |
| 5. Calcium oxide | E: Hygroscopic |
– : End of Water MCQs Class-8 Dalal Simplified Solutions :–
Return to – Dalal Simplified Chemistry ICSE Class-8 Solutions
Thanks
Share with your friends.


