Ammonia Dalal Simplified ICSE Chemistry Class-10 Solutions. Dalal Simplified ICSE Chemistry Ammonia by Dr Viraf and J Dalal for Class 10. Step by step Solutions of Ammonia by Dr Viraf and J Dalal of Concept of Simplified Dalal ICSE Chemistry.
Ammonia Dalal Simplified ICSE Chemistry Class-10
Get Other Chapter Dalal Simplified ICSE Chemistry Class-10 Solutions
How to Solve Mole Concept ICSE Chemistry Class-10
Note:– Before viewing Solutions of Ammonia by Dr Viraf and J Dalal Simplified ICSE Chemistry Solutions .Read the Ammonia Carefully to understand the concept in better way .After reading the Ammonia solve all example of your text book with ICSE Specimen Sample Paper for Class-10 Exam of Council. Ammonia is the Most important Chapter in ICSE Class 10 Chemistry.Previous Year Solved Question Paper for ICSE Board
Additional Questions Ammonia Dalal Simplified ICSE
Question 1.
State why nitrogenous matter produces ammonia. State a liquid source of ammonia.
Answer:
When nitrogenous matter (such as animal and vegetable protiens) decays in the absence of air, the putrefying bacteria on the organic matter in the soil or ammonifying bacteria in organic matter produces ammonia.
The liquid souce of ammonia is decaying urine of animals.
Question 2.
Give the word equation and balanced molecular equation for the laboratory preparation of ammonia from NH4Cl and calcium hydroxide.
Answer:
Ammonium Chloride + Calcium Hydroxide → Calcium Chloride + Water + Ammonia
![]()
Question 3.
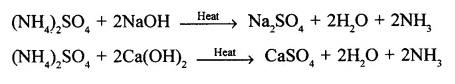
Convert ammonium sulphate to ammonia using two different alkalis.
Answer:
Question 4.
State why ammonia is not obtained in the laboratory from NH4NO3 and NaOH.
Answer:
Ammonium nitrate on heating decomposes explosively with the formation of nitrous oxide and water.
![]()
Question 5.
State the method used with reasons for drying and collecting ammonia gas.
Answer:
Calcium oxide (quick lime) is used for drying ammonia. It is because, calcium oxide being basic in nature does not react chemically with ammonia.
CaO + H2O → Ca(OH)2
Chemicals such as CaCl2 (anhydrous), P2O5 and cone H2SO4 are not used for drying ammonia, because they react chemically with it as shown in the equation below :

Ammonia is collected by downward displacement of air. Ammonia gas is highly soluble in water, as such it cannot be collected over water. Further, ammonia is lighter than air. As such ammonia is collected by downward displacement of air.
Question 6.
State how you would convert (i) Mg (ii) Ca (iii) Al – to ammonia.
Answer:

Question 7.
Give a balanced equation with all conditions to obtain NH3 from N2 and H2.
Answer:
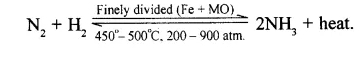
Conditions for maximum yield of ammonia :
- Pressure : 200-900°C (High pressure)
- Temperature : 450-500°C (Optimum temperature)
- Catalyst : Finely divided iron, Fe
- Promotor : Molybdenum, Mo
Question 8.
State two physical properties of NH3 which enable separation of NH3 from a mixture of NH3, N2 and H2.
Answer:
- Ammonia liquifies at a pressure 8 atmosphere at -33°C, but not hydrogen and nitrogen.
- Ammonia is extremely soluble in water, but not hydrogen and nitrogen.
Question 9.
Compare the density of ammonia with that of air. Name two gases lighter than ammonia.
Answer:
The vapour density of ammonia is 8.5 and that of air is 14.4. The two gases lighter than ammonia are
- hydrogen
- helium.
Question 10.
‘Ammonia is highly soluble in water’. Name two other gases showing similar solubility.
Answer:
The other highly soluble gases in water are :
- Hydrogen chloride
- Sulphur trioxide.
Question 11.
Name the experiment and state its procedure to demonstrate the high solubility of ammonia.
Answer:
Highly solubility of ammonia can be shown by Fountain Experiment.
To demonstrate the high solubility of ammonia gas in water.

Apparatus :
- Round bottomed flask filled with ammonia gas.
- Mouth of the flask with a rubber stopper with two holes, one for jet tube and other for dropper containing water.
- Trough below contains red litmus solution.
Procedure :
- The dropper containing water is squeezed and few drops of water enters the flask.
- Ammonia gas present in the flask gets dissolved in water due to its high solubility, which creates a partial vacuum in the flask.
- Since outside pressure is higher, so red litmus solution rush up the jet tube and emerge as a fountain. (Ammonia gas being basic changed red litmus blue.)
Ammonia gas is lighter than air, hence it is collected by downward displacement of air. - Easily liquified at low temperatures.
- Liquid ammonia boils at – 33.5°C
- Solid ammonia melts at – 77.5°C
Question 12.
Give an equation for the burning of ammonia in oxygen. State the observation seen.
Answer:
Ammonia bums in the atmosphere of oxygen with a pale blue flame, forming nitrogen gas and water vapour.
![]()
Question 13.
Convert ammonia to nitric oxide by catalytic oxidation of ammonia. State all conditions.
Answer:
When a mixture of 2 (vols.) of oxygen and l(vols.) of ammonia is passed over platinum gauze maintained at 800°C, it reacts to form nitric oxide and water vapour.
![]()
- Conditions for the reaction : Ostwald’s process
- Temperature : 800°C
- Catalyst : Platinum (Pt)
Question 14.
Draw a simple diagram for the catalytic oxidation of ammonia in the laboratory.
Answer:
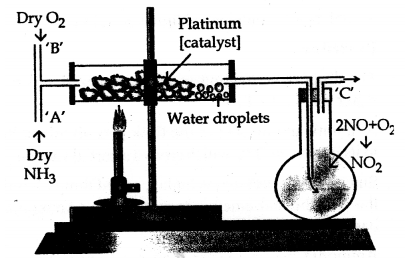
Question 15.
Give reasons for the observation seen during catalytic oxidation of ammonia.
Answer:
The colourless nitric oxide formed undergoes further oxidation to give reddish brown vapours of nitrogen oxide.
The platinum(catalyst) continues to emit a reddish glow even after the heating is discontinued since the catalytic oxidation of ammonia is an exothermic reaction.
Question 16.
Name an industrial process which involves ammonia, oxygen and a catalyst as its starting reactants.
Answer:
The industrial process is called Ostwald’s process for preparing nitric acid.
Question 17.
State what an aqueous solution of NH3 is called. State how it is prepared giving reasons.
Answer:
The aqueous solution of ammonia is chemically ammonium hydroxide(NH4OH) (Liquor Ammonia).
It is prepared by connecting the delivery tube of the apparatus generating ammonia with an inverted funnel whose rim is just dipping in water contained in beaker.
This arrangement provides
- large surface area for the absorption of ammonia and
- prevents back suction
NH3 + H2O → NH4 OH
Question 18.
State why an aq. soln. of NH3
- turns red litmus blue
- is a weak base and a weak electrolyte.
Answer:
- Ammonia on dissolving in water furnishes ammonium (NH4+) ions and hydroxyl (OH–) ions.
![]()
The presence of OH– turns the red litmus blue.
2 When ammonia dissolves in water, it forms ammonium hydroxide. The ammonium hydroxide dissociates to NH4+ ions and OH– ions.
However, the degree of dissociation of ammonium hydroxide molecules is very low. Thus, due to the presence of few OH- ions it is a weak base, as well as weak electrolyte.
Question 19.
State two different methods of preparing NH4Cl using hydrochloric acid.
Answer:
- When ammonium hydroxide is treated with hydrochloric acid, neutrilisation reaction takes place with formation of Ammonium chloride
NH4OH + HCl (dil.) → NH4Cl + H2O - When ammounium carbonate is treated with hydrochloric acid, it forms ammonium chloride, carbon dioxide and water.
(NH4)2 CO3 + 2HCl (dil.) → 2NH4Cl + CO2 + H2O
Question 20.
Convert (i) ammonia (ii) ammonium hydroxide to an ammonium salt using (a) HNO3 (b) H2SO4.
Answer:

Question 21.
State a reason why reaction of liquor ammonia with nitric acid is a neutralization reaction.
Answer:
Liquor ammonia is a saturated solution of ammonia in water. Ammonia, NH3 dissolves in water to give ammonium hydroxide which dissociates partially to give NH4+ and OH– ions. Due to presence of OH– ions, ammonium hydroxide acts as an alkali.
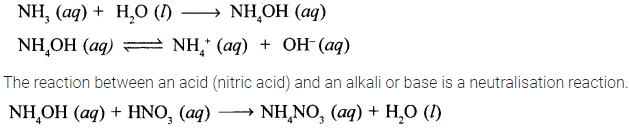
Hence, the reaction between liquor ammonia and nitric acid is a neutralisation reaction.
Question 22.
State why an aqueous solution of ammonia (NH4OH) is used for identifying cations.
Answer:
Because of its colour and solubility in excess NH4OH.
Question 23.
State how NH4OH is used for identify :
- Fe2+
- Fe3+
- pb2+
- Zn2+
- Cu2+ cations.
Give also a balanced equation in each case for a known example.
Answer:

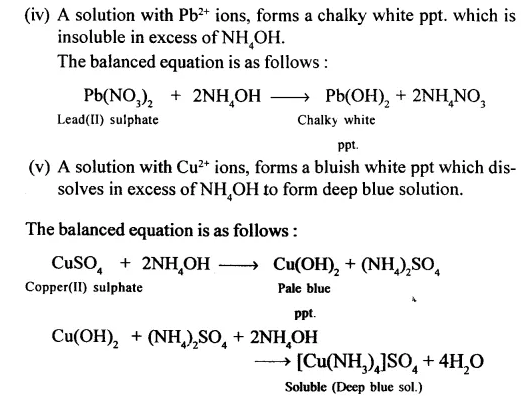
Question 24.
State why the blue ppt. formed on addition of NH4OH to CuSO4 soln. dissolves to give a deep blue solution with excess of NH4OH. Give an equation for the reaction. State why Zn(OH)2 is soluble in excess of NH4OH.
Answer:
CuSO4 when reacts with excess of ammonia it produces needle shape crystals of tetraammine copper(II) sulphate complex which possess a beautiful deep blue colour.
Question 25.
Give balanced equations for the reducing reactions of ammonia with
(i) copper (II) oxide, (ii) lead (II) oxide, (iii) chlorine using, (a) excess NH3, (b) excess Cl2.
Answer:

Question 26.
State five tests for ammonia where a colour change is involved.
Answer:
Tests for ammonia :
- Ammonia turns red (or purple) litmus solution to blue.
- Ammonia turns methyl orange solution to yellow.
- Ammonia turns phenolphthalein (colourless) solution to pink.
- Ammonia turns Nesseler’ reagent (colourless) solution to pale brown.
- Ammonia (when in excess) gives a deep blue coloured solution with CuSO4 (aq.)
Question 27.
State (i) a light neutral gas (ii) an acid (iii) an explosive (iv) a fertilizer — obtained from ammonia.
Answer:
- Hydrogen
- Nitric acid
- NH4NO3
- Ammonium sulphate.
Question 28.
Name an ammonium salt which is a constituent of (a) smelling salts (b) dry cells.
Give reasons for the use of the named ammonium salt for the same.
Answer:
(a) Smelling salts : Ammonium carbonate, (NH4)2CO3 is used as smelling salt. It is an unstable white solid decomposes to give pungent smellingNH3 gas.
(NH4)2CO3 → 2NH3 + CO2 + H2O
The pungent smelling NH3 gas revives a fainted person. (NH4)2CO3 is always kept in a tightly closed container to prevent it from decomposition.
(b) Dry cells : Ammonium chloride(NH4Cl) is used in dry cells. It oxidises Zn to Zn2+ ions. The electrons so produced constitute the electric current.
Question 29.
Give one use with reason of
- an aqueous solution of NH3
- liquefied NH3.
Answer:
(i) Use of an Aqueous solution of Ammonia (NH3) : An aqueous solution of NH3 is called liquor ammonia. Being a base, it can easily emulsify oils and fats. Therefore, it is used for removing oil and fat stains from clothes carpets, upholstery etc. It is also used for cleaning window panes, porcelain articles etc.
(ii) Use of Liqueified Ammonia (NH3) : Liquid ammonia is used as refrigerant i.e. for producing low temperature. This is due to the following reasons:
(a) It is highly volatile.
(b) It can be easily liquefied under high pressure and low temperature.
(c) It’s latent heat of evaporation is very high.
Question 30.
State what are chlorofluorocarbons and give their use. Give a reason why they are ozone depleting. State a suitable alternative to chlorofluorocarbons which are non-ozone depleting.
Answer:
Chlorofluorocarbons (CFC’s) are compounds of carbon with chlorine or fluorine.
CFC are chemicals which like liquefied ammonia gas are used in refrigeration gas. They are also used as coolants in refrigeration and A/c Plants and aerosol sprays and cleansing agents.
These CFC’s deplete ozone layer and also contribute to global warming. Thus these CFC’s are harmful to life. The CFC’s are decomposed by ultraviolet rays coming from sun to produce highly reactive chlorine atoms i.e. free Cl radicals.
Suitable alternatives to chloroflurocarbons which are not depleting ozone are :
HCFC – Hydrochloroflurocarbons; and HFC – 125 Hydrochloroflurocarbons ; which act as a substitute for CFCs, which are non-ozone depleting.
–Try Also :–