Atmospheric Pollution Dalal Simplified Class-9 ICSE Chemistry Solutions Chapter-8. .We Provide Step by Step Answer of Exercise /Lesson -8 Atmospheric Pollution with Additional Questions , Previous Year Questions and Unit Test-8 of Dr Viraf J Dalal Middle School Chemistry Allied Publishers New Simplified Chemistry . Visit official Website CISCE for detail information about ICSE Board Class-9.
Atmospheric Pollution Dalal Simplified Class-9 ICSE Chemistry Solutions Chapter-8
–: Select Topics :–
Previous Year Questions of Exe-Atmospheric Pollution Dalal Simplified Class-9 ICSE Chemistry
Question 1.[2007]
Name any two natural sources of atmospheric pollution.
Answer:
Two natural sources of atmospheric pollution:
- Decay of plants and animals.
- Disintegration of rocks and soil.
Question 2.
Name any two gases which are responsible for the formation of acid rain.
Answer:
SO2 and NO2 are gases responsible for acid rain.
Question 3.
Explain the term ‘global warming’.
Answer:
Global warming: If the percentage of ‘green house gases’ increase, then the surface temperature of earth rises. “The rise in average temperature of earth’s surface is called global warming.”
[2008]
Question 1.
State two effects of ozone depletion.
Answer:
Effects of ozone deletion:
- Ultra-violet rays which are harmful will not be stopped and will reach the earth.
- Many organic species necessary for life will be destroyed.
[2009]
Question 1.
What is meant by the term ‘acid rain’. Give any two impacts of acid rain.
Answer:
Acid rain:
Oxides of sulphur [SO,] and nitrogen [NO,] present air mix with water [or snow, fog etc.] and fall down on the earth resulting in acid rain.
Two impacts of acid rain:
- The water of lakes and rivers which is becoming acidic, may not support aquatic life.
Acid rain damages the buildings and sculptural materials or marble, lime stone, slate, mortar etc. - These materials become pitted and weakened mechanically.
Question 2.
State two ways by which global warming can be reduced.
Answer:
Two ways to reduce global warming:
- We should plant more trees to increase the green cover.
- Minimise the use of automobiles.
Question 3.
State an advantage of using solar energy.
Answer:
Solar energy is renewable source of energy and does not produce pollution.
[2010]
Question 1.
Explain the methods of preventing acid rain.
Answer:
Method of preventing acid rain:
- By using coal or oil – that has low sulphur content. This reduces the emission of oxides of sulphur and nitrogen responsible for acid rain.
- By using scrubbers a device that absorbs gaseous pollutants.
Question 2.
State an advantages of CNG [compressed natural gas].
Answer:
Advantage of using CNG causes less (minimum) pollution. It does not contain lead and lower maintenance cost.
Question 3.
State how CFCs break ozone in the stratosphere.
Answer:
Depletion of 03 (ozone) by CFCs:
C l [g] + O3[g] → ClO[g] + O2[g]
This depletes ozone.
CIO further produces more [Cl] free radical
Cl0[g] + O[g] → Cl[g] O2[g] and destroys more of O3 and there by giving rise of ozone depletion.
Question 4.
Describe the methods of saving the ozone layer.
Answer:
Method to protect ozone layer:
Ozone layer is protected by alternative products like HCFC [hydro chloro fluoro carbons]
Additional Questions of Atmospheric Pollution Dalal New Simplified Class-9 ICSE
Question 1.
State what is meant by the term ‘atmospheric pollution’. Name four gaseous atmospheric pollutants.
Answer:
Atmospheric pollution : Conditions of air made unclean due to introduction of foreign elements to air so as to cause adverse effects on living organisms on earth” is called atmospheric pollution.
Four gaseous pollutants are:
- SO2, SO3
- NO, N02
- CO2, CO
- H2S
Question 2.
Explain the term ‘acid rain’.
State the two forms of deposition of acid rain with suitable examples of each form of deposition.
Answer:
Acid rain: Oxides of sulphur [S02] and nitrogen [N02] present air mix with water [or snow, fog etc.] and fall down on the earth resulting in acid rain.
Two forms are:
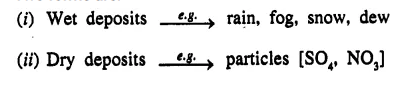
Question 3.
State the natural and man-made sources of the two pollutants responsible for acid rain.
Answer:
Natural sources are:
- Volcanic eruptions
- Forests fires
Man made sources are:
- Automobile exhaust
- Industrial plants
Question 4.
Burning of fossil fuels is an important source of the pollutant – ‘oxides of sulphur’ responsible for acid rain.
State what are ‘fossil fuels’. Name the principal fossil fuels. State why sulphur dioxide is emitted on burning a fossil fuel. Give a balanced equation for the same.
Answer:
Fossil fuels: “Fuels obtained from fossils i.e. organic remains of plants and animals.” Principal fossil fuels : coal, petroleum [oil] and natural gas.
On burning fossil fuels → SO, is produced from sulphur impurities present in coal.
Question 5.
During metallurgy – smelting plants produce sulphur dioxide, when metallic sulphides are roasted in air. Give a balanced equation for the same.
Answer:
When sulphide ores are roasted in smelting plants SO2 is produced:
2ZnS + 3O2 → 2ZnO + 2SO2
Question 6.
State why high temperatures in internal combustion engines release pollutant – oxides of nitrogen.
Answer:
In internal combustion engines combustion occurs with air drawn in. Air in a combustion chamber and this air drawn from the atmosphere contains 80% nitrogen.The nitrogen combines with oxygen at high temperature producing oxides of nitrogen.
N2 + O2 → 2NO [at high temp.]
Question 7.
State why natural rain water produced in an unpolluted atmosphere is slightly acidic. Give a balanced equation of the same.
Answer:
Natural rain water is slightly acidic “as CO2 present in traces in unpolluted air dissolves in rain water forming weak carbonic acid [H2CO3].
Question 8.
Give balanced equations for the formation of sulphuric acid in acid rain, when’ a fossil fuel is burnt in an electric power station.
Answer:
In electric power stations: When coal burns, S present in coal forms
SO2 S + O2 → SO2
SO2 as pollutant in air forms SO3
2SO2 + O2 → 2SO3
This SO3 comes in contact with water vapour present in air to form H2S04 and falls down as acid rain.
Question 9.
Starting from nitrogen in air, enlist the reactions with the help of balanced equations, which result in the conversion of nitrogen in an internal combustion- engine, to the acids formed which are responsible for acid rain.
Answer:
In the internal combustion engine atmospheric air containing 80% N2 is used for combustion of fossil fuel.
At high temp. N2 + O2 → 2NO .
NO is released into air of atmosphere where it forms N02 in presence of lightning discharge.
2NO + 02 → 2NO2
N02 when comes in contact with water vapours to form nitric acid and falls in the form of acid rain
2N02 + H20 →HNO3 + HNO2
2H20 + 4NO2 + 02 → 4HNO3
Question 10.
Explain the basic function of a catalytic converter in an internal combustion engine.
Answer:
The basic function of a catalytic converter is ‘to eliminate pollutants from exhaust gases before they are discharged into the atmosphere’.
Question 11.
State why acid rain causes ‘nutrient leaching’ when it falls on the earth.
Answer:
Acid rain causes ‘nutrient leaching’. H+ – Hydrogen ions produced by the decomposition of acid are added to the soil and interact with the metal. The metal Ca, K etc. are displaced from the earth called nutrient leaching.
Question 12.
Give reasons why acid rain affects marine organisms.
Answer:
Affects marine organisms: Acid rain leach toxic metals like mercury, lead, zinc present in the soil thus toxicty enters rivers and streams and destroys marine organisms.
Question 13.
State the impact of acid rain on the environment, other than soil chemistry and water bodies.
Answer:
Acid rain changes the pH of the environment: Certain species (aquatic animals) are eliminated at pH less than 6 [Salmons die at pH less than 5.3]
Question 14.
Enumerate the ways by which acid rain can be controlled to prevent its adverse effects on the environment.
Answer:
Ways to control acid rain:
- Using catalytic convertors, which can reduce NO emission from automobiles so that NO, and nitric acid is not formed.
- Using C.N.G., Hydro power or wind energy which is cleaner energy and cause less pollution.
Question 15.
State what are ‘green house gases’ and name the major green house gases.
Answer:
Green house gases: “Are those gases that contribute effectively in retaining heat in the atmosphere.”
OR
“The various gases which contribute to green house effect are called green house gases.” Gases are C02, water vapours, oxides of nitrogen, CH4,O3 and chlorofluorocarbons.
Question 16.
State what is mean by the term – ‘green house effect’ and state its consequence.
Answer:
Green house effect:
“Heating of the earth and its environment by carbondioxide and water vapours in the atmosphere is called Green House effect.”
Consequences of green house effect:
- It supports survival of life on earth.
- Plays an important role in evolution of life on earth. Without ‘green house gases’, all the heat coming from sun would have escaped from earth and it would have become cold and barren like moon.
- Increased amount of these gases contribute to global warming.
Question 17.
Explain how global warming takes place in the presence of green house gases. Give a reason why the surface temperature of earth is maintained in absence of green house gases.
Answer:
As light from the sun passes through atmosphere of earth most of the U.V. radiations are absorbed by ozone and only 30% of IR radiations reach earth’s surface and heat it.
As the earth becomes hot, it starts emitting radiations which have now less energy and hence longer wavelength. Some part of reflected IR radiations are absorbed by CO, and this remains on earth and warm up the earth’s surface.
In absence of green house gases surface temperature on earth is maintained. Since heat radiations absorbed after passing clear atmosphere reach earth and some heat., radiations are reflected back.
Question 18.
Give balanced equations for formation of carbon dioxide – a major green house gas, during combustion of fossil fuels.
Answer:
Formation of CO, during:
Combustion of fossil fuels:
C + O2 CO2 → Δ burning of coal.
CH4 + 2O2 → CO2 + 2H2O + Δburning natural gas, .
Question 19.
State two natural and two man-made sources of the green house gas – methane.
Answer:
Sources of CH4 [natural gas]
Natural: (1) Natural gas (2) Marsh gas.
Man made: (1) Coal mining (2) Natural wet lands and rice paddies.
Question 20.
‘Oxides of nitrogen namely nitrous oxide, are released into the atmosphere from varied sources’. Explain the statement with relevant sources.
Answer:
Oxides of nitrogen [N2O] released into the atmosphere from anaerobic respiration, activities of microorganisms in soil release – nitrogen, nitric oxide and nitrous oxide.
Also from –
- Agricultural activities – soil cultivation – use of nitrogenous fertilizers.
- Automobile exhausts.
- Burning of biomass.
Question 21.
State the impact of green house gases on geographic, climatic and agricultural conditions.
Answer:
Impact of green house gases on:
- Geographic conditions is global warming
Increases melting of ice-caps.
Coastal flooding and erosion. - Climatic conditions is tropical regions experience – more rainfall Northern latitudes experience – shorter and wetter winters.
- Agricultural conditions is affects the life cycle of trees and survival and reproduction of plants.
The soil fertility and amount of soil water retained in the soil.
Question 22.
Give the options for reducing green house gases by use of fuels better than fossil fuels.
Answer:
Way of reducing green house gases : use fuels which release-less green house gases.
Like: (1) L.P.G. (2) C.N.G. (3) L.N.G.
C.N.G. cause minimum pollution. It does not contain lead and lowers maintenance cost.
[But these are also fossil fuels]
Use solar energy, wind energy, hydro-power, hydrogen energy, biofuels and biogas.
Question 23.
State three renewable energy sources which cause less or no pollution.
Answer:
Renewable sources of energy are:
- Solar energy
- Wind energy
- Hydropower energy cause no pollution.
Question 24.
Explain how promoting ‘afforestation’ and checking ‘deforestation’ reduces the release of green house gases.
Answer:
Afforestation growing more plants and forest help to absorb green house gases CO2 during photosynthesis
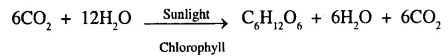
Checking deforestation means reduce burning of wood and also use of bio fertilizers instead of nitrogenous fertilizers help control of emission of green house gases i.e. nitrous oxide.
Question 25.
What is an ozone layer. State the distribution of the ozone layer in the stratosphere.
Answer:
Ozone layer: “Is a layer in the earth’s atmosphere which contains relatively high concentration of ozone [O3].” Ozone layer is distributed 20-30 kms above the earth in stratosphere.
Question 26.
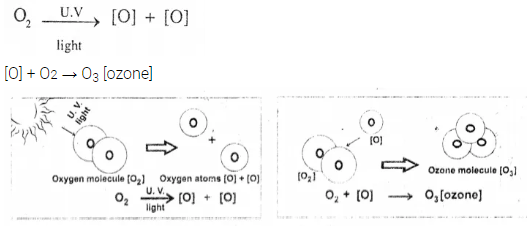
Give balanced equations for formation of ozone molecules from oxygen molecules. What type of reaction takes place, during the formation.
Answer:
The reaction that takes place during the formation is photolysis or photo dissociation. Highly energetic UV from the sun break the O2 molecule in two atoms. Highly reactive O atom reacts with O2molecule to from ozone O3
Question 27.
How is the ‘ozone oxygen cycle’ maintained in the stratosphere and state what disturbs the balance, resulting in ozone depletion or destruction, Give balanced equation for the same.
Answer:
Ozone-oxygen cycle: “Breaking of ozone into O2 in the stratosphere and making of ozone from oxygen in stratosphere and to maintain total concentration of ozone almost the same is called ozone-oxygen cycle.” Breaking of O2 to form O3.
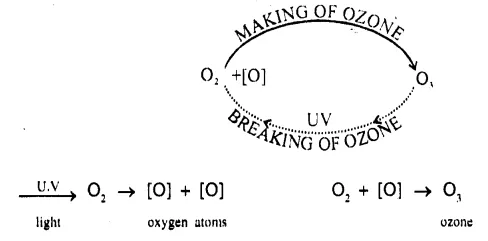
Photolysis : 02 [molecule] in presence of U.V. from sunlight in stratosphere breaks into [0] atom [very reactive].
03 is broken down [with the help of U.V. rays] into O2 + [O]
U.V. rays → O3 → O2 + O
O + [O] → O2molecules and O3 + [O] → 2O2
Very reactive oxygen atoms
Depletion or destruction of ozone is due to increased level of [Cl], [Br], [NO], [OH] free radicals
These free radicals are highly reactive and attack 03 and deplete 03
Cl[g] + O3[g] → C10[g] + O2
C10[g] + O[g] →4 Cl[g] + 0,[g]
NO + O3→4 NO2 + O3
NO2+ O3 → NO3 + O2
In this way cause depletion of ozone.
Question 28.
Enumerate the damage to humans, animals and plants caused due to ozone depletion.
Answer:
Damage cause due to ozone depletion to humans:
- Sun burn and skin cancer
- Disruption in function of DNA
- Early aging of skin
- weakening of immune system
- eye cataract and eye damage
Animals: Severe damage to cells of animals and all the damages caused to humans as above.
Plants:
- Retards plant growth.
- Reduces chlorophyll content
- inhibits pollen germination
- Disrupts ecosystem, quality of vegetables
- increases harmful mutations
Question 29.
Name the chemicals responsible for destruction of the ozone layer. State the main chemical from these chemicals, which is responsible for more than 80% ozone depletion.
Answer:
Chemicals responsible for ozone depletion are: [CFCs] chlorofluoro carbons, CR,CL, CH3Br, CCl4, CH4 and nitrous oxide [N2O]
Main responsible chemical is chlorofluorocarbons CFCl, for 80% depletron.
State the man-made applications which make use of that chemical.
Man made application is coolant in refrigeration and A.C.’s
Question 30
State the role of chlorofluorocarbons in ozone destruction or depletion [no equations required].
Answer:
role of chlorofluorocarbons in ozone destruction or depletion
Roll of chlorofluorocarbons in ozone destruction : Chlorofluorocarbons are decomed by U.R. rays and liberate highly reactive free CF radical. This free ladical reacts with ozone to form Cl0[g]. This causes ozone depletion. The Cl free radicals initiate and catalyse a chain reaction – capable of breakdown of over 1,00,000 ozone molecules resulting in ozone depletion.
Question 31.
Enumerate methods of protecting the ozone layer and preventing its depletion.
Answer:
Methods to protect ozone layer:
- By replacing CFC’s by alternative products and use of HCFC hydochloro fluoro carbons.
- International treaty [Montreal protocol] was also initiated to prevent ozone depletion.
UNIT TEST PAPER 8 —Atmospheric Pollution Dalal New Simplified Class-9 ICSE Chemistry
l. Select the correct answer from the choice A, B, C give in each case.
1.The major pollutant released during burning of fossil fuels.
A: Carbon monoxide
B: Sulphur dioxide
C: Hydrogen sulphide
Answer
Sulphur dioxide
2 .The green house gas which on combustion produces another green house gas.
A: Nitrous oxide
B: Ozone
C: Methane [CH] produces CO2
Answer
Methane [CH] produces CO2
3. The gas which in presence of U.V. light gives two atoms of the same gas.
A: Oxygen
B: Ozone
C: Carbon dioxide
Answer
Oxygen
4. A chemical responsible for ozone depletion.
A: Methyl acetylene
B: Methyl chloride
C: Methanol
Answer
Methyl chloride
5. A renewable source of energy which causes minimum or no pollution.
A: Fossil fuel
B: L.P.G.
C: Hydro power.
Answer
Hydro power.
2. Give balanced equations for the following conversions [one or two steps].
- Sulphur trioxide to sulphuric acid – a constituent of acid rain.
- Nitrogen to nitrogen dioxide – in an internal combustion engine.
- Methane to carbon dioxide – a green house gas.
- A molecule of ozone to two molecules of oxygen gas.
- Oxygen to ozone gas by photolysis.
Answer:
1.SO3 to H,S04
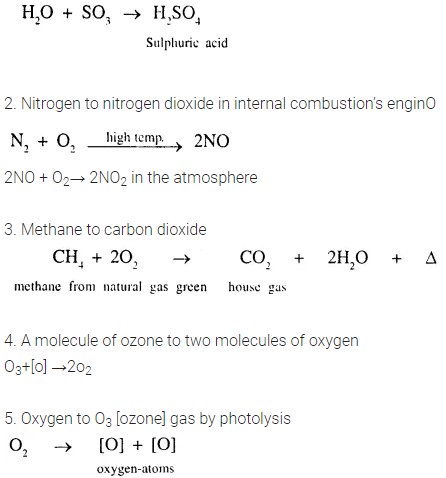
3. Give reasons for the following.
1. Natural rain water does not have a pH of 7 [i.e. neutral]
Ans. Natural rain water is acidic in nature due to acid gases dissolved and is not neutral i.e. pH of 7.
2. A catalytic converter in an internal combustion engine reduces pollution.
Ans: Catalytic converters reduce the nitrogen oxide emission from automobiles or devices that can absorb-gaseous pollutants.
3. In absence of green house gases the surface temperature of the earth is maintained.
Ans: In absence of green house gases the surface temp, of earth is maintained as from sun the heat radiations reach earth and are absorbed and retained here.
Some of the absorbed radiations are reflected back into the atmosphere. Hence balance is maintained between heat energy absorbed by earth and radiated from the earth.
4. The formation of ozone involves a chemical reaction called photolysis.
Ans: With the help of UV rays
02 molecule breaks into two oxygen atoms called photolysis.
Now more of O2 molecule combine with [O]
02 + [O] → O3 ozone
5. Destruction of ozone layer is harmful for both humans and plants.
Ans: For answer see Q. 28.
4. Name or state the following:
- An atmospheric pollutant produced during lightening discharge.
Ans: Oxide of nitrogen NO, NO4 . - A form of wet deposition of acid rain other than rain water.
Ans: Snow, dew. - An atmospheric pollutant responsible for both global warming and ozone depletion.
Ans: CH4 [methane]. - A green house gas which contains carbon and hydrogen only.
Ans: CH4 [methane] - The atom which reacts with oxygen to form ozone.
Ans: [O] oxygen atom.
5. Acid rain has adverse effects on the environment State the effect acid rain has on the following
- A: Increases or B: Decreases.
- The nutrients present in the soil.
- The acidity of the soil.
- The fertility of the soil.
- The pH of water bodies.
- The rate of photosynthesis in plants.
Answer:
- Causes nutrient leaching i.e. they are displaced from the soil. (Decreases)
- Acidity of soil increases.
- Soil fertility is reduced. (Decreases)
- Changes the pH of the water bodies. (Decreases)
- The rate of photosynthesis in plants is reduced. (Decreases)
6. The diagram represents the green house effect.
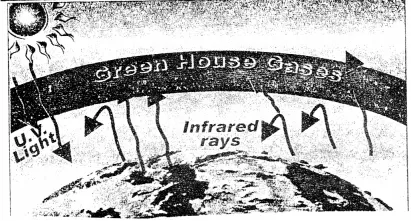
- State why the effect is called ‘global warming’.
- Name a process which (a) releases (b) absorbs – green house gas – CO2.
- State an advantage of use of C.N.G. over combustion of fossil fuels.
- Which of the following – biofertilizers or nitrogenous fertilizers reduces the green house gas – CO2. Explain.
- Does the sea level rise or fall due to global warming. Explain.
Answer:
- In trapping of UV rays by CO2 i.e. green house gas results in an average rise in atmospheric temperature and has a global impact but not confined to one region. It is called global warming.
- Process which releases – CO2 is respiration process which absorbs – CO2 is photosynthesis.
- C.N.G. – it causes minimum pollution. It also does not contain lead and lower maintenance cost compared to vehicles with other forms of fuels.
- Biofertilizers – help in grow more plants which absorb (consume) more CO2 for photosynthesis and help to reduce green house gas – CO2.
- The sea level rises due to global warming as increase in temperature melts ice on mountains, ice-caps.
.– : End of Atmospheric Pollution Dalal Simplified Solutions :–
Return to New Simplified Dalal ICSE Chemistry Class-9 Solutions
Thanks
Share with your friends



Very helpful answers for me and I hope that rest will also like it but from my side it really helped me a lot…..thank you so so much for your kind Answers
thanks