Atomic Structure and Chemical Bonding Exe-2 Goyal Brother ICSE Class-9 Ch-4 Solutions. We Provide Solutions of Chapter-4 Exercise-2 Atomic Structure and Chemical Bonding Goyal Brother Prakashan ICSE Class-9 Ch-4. Visit official Website CISCE for detail information about ICSE Board Class-9.
Ch-4 Atomic Structure and Chemical Bonding Exe-2 Goyal Brother ICSE Class-9
| Board | ICSE |
| Publications | Goyal Brother Prakashan |
| Subject | Chemistry |
| Class | 9th |
| Writer | Dr. S.K. Aggarwal |
| Chapter-4 | Atomic Structure and Chemical Bonding |
| Topics | Solutions of Exercises-2 |
| Edition | for 2022-2023 Academic Session |
Ch-4 Atomic Structure and Chemical Bonding
Goyal Brother Prakashan ICSE Class-9 Chemistry Solutions
(Page- 101-103)
Questions 1.
(a) What do you understand by the term valence electrons?
(b) why are valence electrons named as such, rather than only electrons?
Answer :
(a) The electrons present in the valence shell are called valence electrons
(b) Because these outer shell electrons are responsible in chemical bonding
Questions 2. An element is represented as 40X20. . Name the shell which has valence electrons and the number of valence electrons in it.
Answer : Atomic number =20
Electronic configuration = 2, 8, 8, 2
Name the shell which has valence electrons= N shell
and the number of valence electrons in it.= 2
Questions 3. Amongst the electrons revolving around the nucleus, which electrons will (a) determine chemical properties of an element? (b) do not determine chemical properties of an clement? Give a reason for your answer.
Answer : a) d Valence electron
(b) other than valence shell
Questions 4. If elements P, Q , R and S have same number of valence electrons, will they have same kind of chemical properties? Support your answer by one example.
Answer : Yes due to same valency
Questions 5. Fill in the blank spaces with appropriate words:
(a) The electrons present in the ..Outer. shell of an atom are called valence electrons.
(b) The electrons present in a shell, other than…Valence Shell… shell do not take part in chemical…reaction.
(c) The nature of an element is if it has one to three ….Valence…. electrons.
(d) An element is a …Inert… gas, if it has…. 8 … valence electrons.
(e) Element….Hydrogen….. has one electron in K-shell but is a non-metal.
(f) Element …Helium……. has two electrons in K-shell, but is a noble gas.
Questions 6. Atomic numbers of elements P, Q, R and S are 1, 2, 7 and 11 respectively. Classify them as metals, non-metals and noble gases.
Answer : P and R are non metal, Q is noble gas S is metal
Questions 7. An element is represented by symbol X?
(a) What is the mass number and atomic number of element X?
(b) Name its valence shell and number of electrons in the valence shell.
(c) Is the clement a metal or a non-metal?
Answer : update soon
(a)
(b)
(c)
Questions 8. Three elements P, Q and R have atomic numbers 4, 12 and 20 respectively.
(a) State the number of valence electrons in each of the elements
(b) Are these elements going to have similar or different chemical properties?
(c) Are these elements metals or non-metals?
Answer :
(a) number of valence electrons in P, Q and R is 2, 2, 2 respectively
(b) similar chemical properties?
(c) these elements metals
Questions 9.
(a) Name an element which has one valence electron, but is a non-metal. —- Hydrogen
(b) Name an element which has two valence electrons, but is a noble gas. — Helium
Questions 10. State the number of valence electrons in (a) alkali metals and (b) halogens.
Answer : valence electrons in (a) alkali metals are 1 and (b) halogens are 7
Atomic Structure and Chemical Bonding Exe-2 Goyal Brother ICSE Class-9 Ch-4 Solutions.
Questions 11. Atomic numbers of elements A, B, C and D are 3, 9, 11 and 17 respectively. Identify two pairs amongst the elements which have similar chemical properties. Which pair is likely to be metallic in character?
Answer : we know that chemical properties depend upon number of valence electron hence first find out valence electron
A3=2,1,
B9=2,7
C11=2,8,1
D17 = 2,8,7
Therefore Element A, C and B, D are two pair
A,C pair is metallic
Questions 12. What do you understand by the term isotope?
Answer : An isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in the periodic table and nearly identical chemical behavior but with different atomic masses and physical properties. Every chemical element has one or more isotopes.
Questions 13. What is the main cause of existence of isotopes?
Answer : The reason for the existence of isotopes is a change in (c) neutron number. A given element can have different isotopes owing to the difference in the number of neutrons but the number of protons and electrons remains the same.
Questions 14. Name three isotopes of hydrogen, give their symbols, number of protons and number of neutrons.
Answer : They each have one single proton (Z = 1), but differ in the number of their neutrons. Hydrogen has no neutron, deuterium has one, and tritium has two neutrons. The isotopes of hydrogen have, respectively, mass numbers of one, two, and three. Their nuclear symbols are therefore 1H, 2H, and 3H
Questions 15. Why do the isotopes of same elements have similar chemical properties?
Answer : They have similar chemical properties because isotopes of an element have the same number of electrons as an atom of that element. The electron arrangement is the same owing to same chemical properties. However they have different numbers of neutrons, which affects the mass number
Questions 16. Why do all elements have fractional atomic mass?
Answer : The atomic masses of most elements are fractional because they exist as a mixture of isotopes of different masses. Since the atomic mass of isotopes are different, so average atomic mass is calculated for a single element.
Questions 17. The ratio of isotopes of 35Cl17. and 37Cl17 in natural chlorine is 1: 3. Calculate the fractional atomic mass of chlorine.
Answer : Atomic mass of chlorine
Questions 18. Fill in the blank spaces with appropriate words:
(a) Isotopes have different ..Mass… number, because their nuclei contain different number of …Neutrons…….
(b) Isotopes of an element have …..Same… chemical properties, because they have same number of ….Proton.. in their nucleus.
(c) Some carbon atoms have mass number 12 and some have mass number 14, These atoms of different mass numbers are called ..Isobars.. .
Questions 19. An atom A has the mass number 40 and atomic number 18. An atom B has the mass number 40 and atomic number 20.
(a) What is the electronic configuration of A?
(b) What its the electronic configuration of B?
(c) Are A and B isotopes of the same clement?
(d) Which amongst A and B is a metal?
Answer :
(a) electronic configuration of A is = 2,8,8
(b) electronic configuration of B is= 2,8,8,2
(c) No Isotope due to deferent atomic number
(d) B is metal
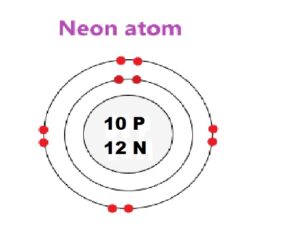
Questions 20. 20Ne10, 21Ne10 and 22Ne10 are the isotopes of neon.
(a) What do the subscripts and superscripts represent
(b) What is the cause of change in superscripts?
(C) Give the geometric diagram of 22Ne10
Answer :
(a) subscripts represent Atomic number and superscripts atomic mass
(b) Difference number of neutrons in nucleus
(c) geometric diagram of 22Ne10
Atomic Structure and Chemical Bonding Exe-2 Goyal Brother ICSE Class-9 Ch-4 Solutions.
Questions 21. Give one similarity and one difference between the isotopes 14C6 and 12C6.
Answer :
Similarities:
- They all have the same number of protons
- They all have the same number of electrons
- They all have the same valence arrangement.
- They all react the same in chemical reactions
Differences
- The all have different number of neutrons
- They all have different atomic weights.
- They all have different percent abundances.
Questions 22. 1H1, 2H1 and 3H1 are the isotopes of hydrogen.
(a) Why do these isotopes have similar chemical properties?
(b) Why are these isotopes electrically neutral?
Answer :
(a) They have similar chemical properties because isotopes of an element have the same number of electrons as an atom of that element. The electron arrangement is the same owing to same chemical properties
(b) In every isotope the charged subatomic particles which are protons and electrons are in equal number. Thus, an isotope is electrically neutral. Note: In every isotope there will be an equal number of electrons and protons
Questions 23. Explain why 3He2 and 3H1 are not considered as isotopes.
Answer : 3He2 and 3H1 are not considered isotopes because they have different atomic number but isotopes have same atomic number and different mass number
Questions 24. The composition of two atoms P and Q given below:
| Atom | Neutrons | Protons | Electrons |
| P | 13 | 11 | 11 |
| Q | 12 | 11 | 11 |
(a) What is the mass number of P?
(b) What is the mass number of Q?
(c) How are and Q related to each other ?
(d) which clement do these atoms represent?
(e) Are the elements metals or non-metals?
Answer :
(a) mass number of P= 13+11=24
(b) mass number of Q= 12+11= 23
(c) Q and P are isotope
(d) Sodium
(e) Metals
Questions 25. The table below shows the elements from P to T, such that a pair of elements is an isotope. Identity the pair and give a reason for your choice:
| Elements | No. of protons | No. of neutrons | No. of electrons |
| P | 11 | 12 | 11 |
| Q | 12 | 12 | 12 |
| R | 11 | 13 | 11 |
| S | 13 | 14 | 13 |
| T | 14 | 14 | 14 |
Answer : we know that Isotope have same atomic number hence same Number of Proton therefore P and R are Isotope
— End of Atomic Structure and Chemical Bonding Exe-2 Goyal Brother ICSE Class-9 Ch-4 Solutions. :–
Return to: ICSE Class-9 Chemistry Goyal Brothers Prakashan Solutions
Thanks


