Atomic Structure and Chemical Bonding Exe-4E Structured Answer Chemistry Class-9 ICSE Selina Publishers Solutions Chapter-4. Step By Step ICSE Selina Concise Solutions of Chapter-4 Atomic Structure and Chemical Bonding with All Exercise including MCQs, Very Short Answer Type, Short Answer Type, Long Answer Type, Numerical and Structured/Application Questions Solved . Visit official Website CISCE for detail information about ICSE Board Class-9.
Atomic Structure and Chemical Bonding Exe-4E Structured Answer Chemistry Class-9 ICSE Concise Selina Publishers
| Board | ICSE |
| Publications | Selina Publication |
| Subject | Chemistry |
| Class | 9th |
| Chapter-4 | Atomic Structure and Chemical Bonding |
| Book Name | Concise |
| Topics | Solution of Exercise – 4E Structured Answer Type |
| Academic Session | 2023-2024 |
Exercise – 4E Structured Answer Type
Atomic Structure and Chemical Bonding Class-9 Chemistry Concise Solutions
Page-82
Question 1.
The atom of an element A has 6 electrons in its M shell.
(a) Write the electronic configuration of element A.
(b) What is the atomic number of element A?
(c) Is it a metal or a non-metal?
(d) What type of ion will be formed by an atom of element A? Write the symbol of the ion formed.
(e) What could element A be ?
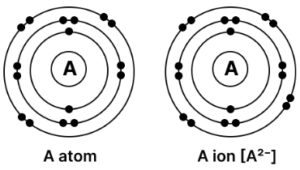
(f) Draw the orbital structure of A and its ion.
Answer:
(a) [2, 8, 6], as it has three shells — K, L, M.
(b) 16, as atomic number is the total number of electrons so, 2+8+6 = 16
(c) Non-metal, as it has 6 electrons in the valence shell and will accept 2 electrons to complete its octet.
(d) Anion, A2-, as element A has 6 valence electrons so it has a valency of -2 and it will take two electrons, complete its octet and become a negatively charged ion.
(e) Sulphur, as sulphur also has the electronic configuration of [2, 8, 6].
(f) Orbital structure of A and its ion is shown below:
Question 2.
An atom of an element Y may be written as 94Y.
(a) What does the figure 9 indicate?
(b) What does the figure 4 indicate?
(c) What is the number of protons in atom Y?
(d) What is the number of neutrons in atom Y?
(e) What is the number of electrons in atom Y?
(f) How many electrons are there in the outermost shell of an atom of element Y?
(g) Write the symbol of ion formed by an atom of element Y?
Answer:
(a) Mass number is indicated by figure 9, which is the sum of protons and neutrons in the nucleus of an atom.
(b) Atomic number is indicated by figure 4, which is the number of electrons present in a neutral atom.
(c) 4, as the atomic number also indicates the number of protons in the nucleus of an atom.
(d) 5, number of neutron = mass number – number of protons = 9-4=5
(e) 4, as the atomic number is 4, which is the number of electrons present in a neutral atom.
(f) 2, its electronic configuration is [2, 2]
(g) Y2+, as it has the valency of +2, hence it loses two valence electrons and becomes a positively charged ion.
Question 3.
Atom A is represented as 20982A, atom B as 20983B and atom C as 21182C
(a) How many electrons, protons and neutrons do A, B and C have?
(b) Which two atoms have the different number of nucleons?
(c) Name the term which can be used for A and C atoms.
(d) Define the term mentioned in part (c) above.
Answer:
(a)
| Atom | Electron (e) | Proton (p) | Neutron (n = mass number – p) |
|---|---|---|---|
| 20982A | 82 | 82 | 209 – 82 = 127 |
| 20983B | 83 | 83 | 209 – 83 = 126 |
| 21182C | 82 | 82 | 211 – 82 = 129 |
(b) A and C have different number of nucleon, because the number of protons is same in both but number of neutrons are different.
(c) Isotopes
(d) Isotopes are atoms of the same element having the same atomic number but different mass numbers.
Question 4.
(a) Name the charged particles which attract one another to form electrovalent compounds.
(b) In the formation of electrovalent compounds, electrons are transferred from one element to another. How are electrons involved in the formation of a covalent compound?
(c) The electronic configuration of nitrogen is (2, 5). How many electrons in the outer shell of a nitrogen atom are not involved in the formation of a nitrogen molecule?
(d) In the formation of magnesium chloride (by direct combination between magnesium and chlorine), name the substance that is oxidized and the substance that is reduced.
Answer:
(a) Cation and anion
(b) By mutual sharing of electrons
(c) Two
(d) Magnesium is oxidized and chlorine is reduced.
Question 5.
An element X has 2 electrons in its M shell, it forms bond with an element Y which has 7 electrons in its third orbit.
(a) Write the formula of the compound formed.
(b) Which nearest inert gas electronic configuration will element X and Y acquire.
(c) Show by orbital diagram the formation of compound between X and Y.
Answer:
Element X has 2 electrons in its M shell.
The electronic configuration would be 2, 8, 2.
So, element X is Mg (12).
Element Y has 7 electrons in its third orbit.
The electronic configuration would be 2, 8, 7.
So, element Y is Cl (17).
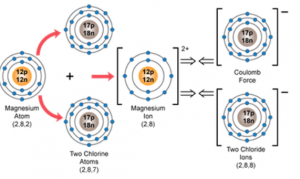
(a) MgCl2
(b) The nearest inert gas electronic configuration for element X is 2,8, while that for element Y is 2,8,8.
(c) Orbital diagram showing the formation of the compound between X and Y:
Magnesium chloride:
Question 6.
In the formation of (i) oxygen molecule (ii) carbon tetrachloride molecule, state the following:
(a) Electronic configuration of nearest inert gas attained.
(b) How many electrons are shared/transferred in bond formation
(c) Which type of bonds these compounds form?
(d) Draw their orbital diagrams.
Answer:
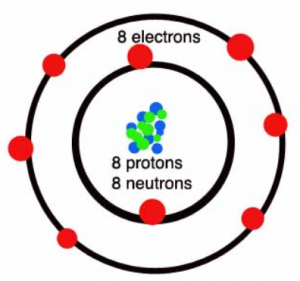
In the formation of Oxygen molecule
(i) oxygen molecule
(a) Neon (10) 2,8
(b) Two pairs of electrons are shared.
(c) Covalent bond
(d) Orbital Diagram of Oxygen molecule
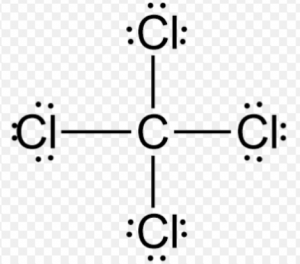
(ii) Carbon tetrachloride molecule
(a) Neon (10) 2,8
(b) Four pair of electrons are shared.
(c) Covalent bond
(d) Orbital Diagram:
— : End of Atomic Structure and Chemical Bonding Exe-4E Structured Answer Class-9 ICSE Chemistry Solutions :–
Return to Return to Concise Selina ICSE Chemistry Class-9
Thanks
Please share with your friends


