Atoms, Molecules and Radicals ICSE Class-7th Concise Selina Chemistry Solutions Chapter-4. We Provide Step by Step Answer of Objective, True False , Fill in the blanks, Match the following , Short/Long Answer Type of Exercise-4 Atoms , Molecules and Radicals . Visit official Website CISCE for detail information about ICSE Board Class-7.
Atoms Molecules and Radicals ICSE Class-7th Concise Selina Chemistry Solutions
EXERCISE-4
Question 1.
Define the following terms :
Answer:
- Atom : An atom is the smallest indivisible unit of an
OR
Atom is the smallest unit of matter. - Molecule : Molecule is the smallest unit of a compound (or an element) which always has an independent existance.
- Radicals : A radical is an atom of an element or a group of atoms of different elements that behaves as a single unit with a positive or negative charge on it.
- Valency : It is the number of electrons donated or accepted by the valence shell of an atom during chemical combination.
- Periodic table represents the tabular arrangment of elements in horizontal rows called periods and vertical columns called groups in order to classify the elements and their systematic study.
Question 2.
Write the names of the elements present in the following compounds.
Answer:
- Common salt : Sodium, chlorine.
- Ammonia : Nitrogen, hydrogen.
- Sulphuric acid : Hydrogen, sulphur, oxygen.
- Glucose : Carbon, hydrogen, oxygen.
- Sodium hydroxide : Sodium, oxygen, hydrogen.
- Acetic acid : Carbon, hydrogen, oxygen.
Question 3.
What does each of the following represent ?
Answer:
- 2C02 = 2 molecules of carbon dioxide.
- 2H2S = 2 molecules of hydrogen sulphide.
- 5H2S04 = 5 molecules of sulphuric acid.
- 6NaNO3 = 6 molecules of sodium nitrate.
Question 4.
Write the symbols and valencies of the following radicals:
Answer:

Question 5.
Name the following radicals :
Answer:
- SO42- = Sulphate
- HC03– = Bicarbonate
- OH- = Hydroxide
- Cr2072- = Dichromate
Question 6.
- Name one ion for each of the valencies +1, +2 and +3.
- Name one ion for each of the valencies-1, -2 and -3.
Answer:
- +1 = Sodium Na+
+2 = Calcium Ca+2
+3 = Aluminium Al+3 - -1 = Chlorine Cl-
-2 = Oxygen 0-2
-3 = Nitrogen N3_
Question 7.
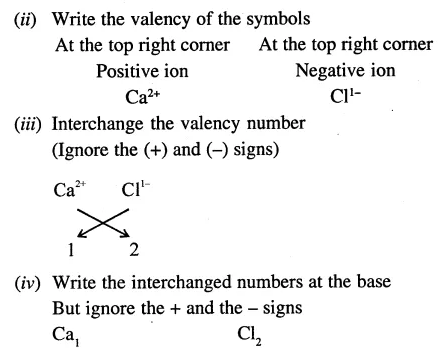
The valency of calcium is 2. Write the valencies of other radical in the following :
- CaO
- Ca(OH)2
- CaC03
- CaCl2
Answer:
- O= 2
- OH = 1
- CO3 = 2
- Cl = l
Question 8.
Write the names of the following compounds :
Answer:

Question 9.
Write the molecular formulae of:
Answer:
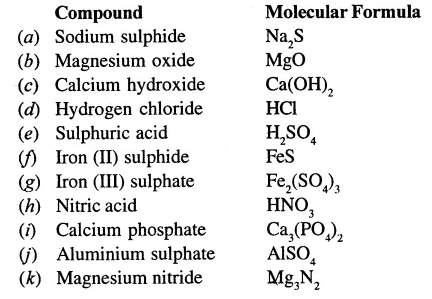
Question 10.
The valency of sodium is one, write the molecular formula for the following compounds of sodium.
- sodium oxide : Na20
- and sodium sulphate : Na2S04
- sodium carbonate : Na2CO3
- hence sodium hydroxide : NaOH
- sodium nitrate : NaN03
Question 11.
What is variable valency ? Give two examples of elements showing variable valency.
Answer:
There are some elements with more than one valency. They are said to have variable valency, e.g. Iron, copper.
Question 12.
Give the group number of following elements present in periodic table
- Magnesium : IIA
- Carbon : IVA
- Sulphur : VIA
- Neon : Zero
Question 13.
An element belongs to group VA. What would be its valency? Name two such elements.
Answer:
Elements of group VA has valency 3.
Two elements : Nitrogen and phosphorus.
Question 14.
An element belongs to group II. What would be its valency? Write the formula of molcules of compounds it will form with elements in VA, VIA and VIIA groups.
Answer:
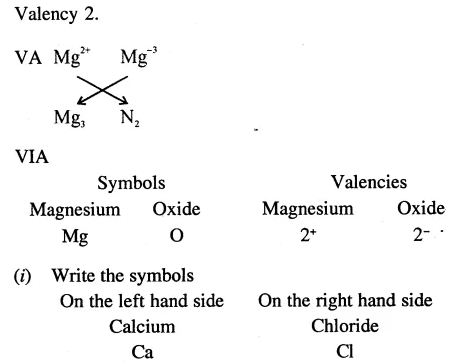
OBJECTIVE TYPE QUESTIONS
Fill in the blanks:
1 .Atoms are …….
Atoms are neutral.
2 .An ion with positive charge is called …….
An ion with positive charge is called cation.
3 .An ion with negative charge is called ……..
An ion with negative charge is called anion.
4 .2H2 means two …….of hydrogen.
2H2 means two atoms of hydrogen.
5. …… is a triatomic molecule.
Ozone is a triatomic molecule.
6. Metals have…….. valency.
Metals have variable valency.
7 .Chemical name of caustic soda is ………………………
Chemical name of caustic soda is sodium hydroxide NaOH.
2. Tick (√) the correct answer.
a) The valency of iron in Fe203 is
- 1
- 2
- 3
- 6
Answer:
3
(b) Which of the following has valency 4 ?
- aluminium
- oxygen
- carbon
- phosphorus
Answer:
phosphorus
(c) The sulphate radical is written as S042-. What is the formula of calcium sulphate ?
- Ca(S04)2
- Ca2(S04)
- Ca(S04)3
- CaS04
Answer:
- CaS04
(d) Which of the following exhibit variable valency ?
- calcium
- copper
- carbon
- chlorine
Answer:
copper
3. State the term for the following:
- The number of atoms present in a molecule of an element atomicity.
- The symbolic representation of a molecule molecular formula.
- A group of atoms that react as a single unit molecule.
- The combining capacity of an element valency.
- The tabular arrangement of elements in horizontal rows and vertical columns periodic table.
Return To ICSE Class – 7 Concise Selina Chemistry Solution
Thanks