Carbon and Its Compounds ICSE Class-8th Concise Selina Chemistry Solutions Chapter-9. We Provide Step by Step Answer of Objective, True False , Fill in the blanks, Match the following , Short / Long Answer Type of Exercise-9 Carbon and Its Compounds. Visit official Website CISCE for detail information about ICSE Board Class-8.
Carbon and Its Compounds ICSE Class-8th Concise Selina Chemistry Solutions
Exercise – I
Question 1.
Fill in the blanks.
(a) Carbon is present in both living and non-living things.
(b) The tendency of an element to exist in two or more forms but in the same physical state is called Allotropy.
(c) Crystalline and non- crystalline are the two major crystalline allotropes of carbon.
(d) Diamond is the hardest substance that occurs naturally.
(e) The name ‘carbon’ is derived from the Latin word carbo.
Question 2.
Choose the correct alternative.
(a) In combined state, carbon occurs as
(i) coal
(ii) diamond
(iii) graphite
(iv) petroleum
Answer:
petroleum
(b) A crystalline form of carbon is
(i) lampblack
(ii) gas carbon
(iii) sugar
(iv) fullerene
Answer:
sugar
(c) Graphite is not found in
(i) Bihar
(ii) Maharashtra
(iii) Orissa
Answer:
Maharashtra
(d) Diamond is used for
(i) making the electrodes of electric furnaces.
(ii) making crucible for melting metals.
(iii) cutting and drilling rocks and glass.
(iv) making carbon brushes for electric motors.
Answer:
cutting and drilling rocks and glass
(e) Carbon forms innumerable compounds because
(i) it has four electrons in its outermost shell.
(ii) it behaves as a metal as well as a non- metal.
(iii) carbon atoms can form long chains.
(iv) it combines with other elements to form covalent compounds.
Answer:
carbon atoms can form long chains.
Question 3.Carbon and Its Compounds ICSE
Write ‘true’ or ‘false’ against the following statements.
(a) Carbon constitues 0.03% of the earth’s crust. – True
(b) Graphite is the purest form of carbon. – False
(c) Coloured diamonds are costlier than colourless and transparent diamonds. – False
(d) Graphite has layers of hexagonal carbon bondings. – True
(e) Diamond is insoluble in all solvents. – True.
Question 4.
Define the following terms:
(a) Allotropy (b) Carat
(c) Crystal (d) Catenation
Answer:
(a) Allotropy: Allotropy is defined as the phenomenon due to which an element exists in two or more forms in the same physical state with identical chemical properties but with different physical properties.
(b) Carat – The weight of diamond is expressed in carats [ 1 carat = 0.2 g]
(c) Crystal – A crystal is a homogeneous solid which particles (atoms, molecules or ions) are arranged in difinite pattern due to which they have definite geometrical shape with plane surfaces e.g. sugar and sodium chloride.
(d) Catenation – The large number of organic compounds is due to the ability of carbon atom to form long chains with other carbon atoms through the sharing of electrons. This unique property of carbon is known as catenation.
Question 5.
State the terms:
(a) Substances whose atoms or molecules are arranged in a definite pattern. – Crystals.
(b) Different forms of an element found in the same physical state. – Allotropy.
(c) The property by which atoms of an element link together to form long chain or ring compounds. – Catenation
Question 6.Carbon and Its Compounds ICSE
Name the following:
(a) The hardest naturally occurring substance. – Diamond.
(b) A greyish black non- metal that is a good conductor of electricity. – Graphite.
(c) The third crystalline form of carbon. – Fullerenes.
Question 7.
Answer the following questions:
(a) Why is graphite a good conductor of electricity but not diamond?
(b) Why is diamond very hard?
(c) What are fullerenes? Name the most common fullerenes.
(d) What impurity is present in black diamond?
(e) Explain the softness of graphite with reference to its structure.
Answer:
(a) In a graphite molecule, one valence electron of each carbon atom remains free, Thus making graphite a good conductor of electricity. Whereas in diamond, they have no free mobile electron. Thats why diamond are bad conductor electricity.
(b) A diamond is a giant molecule. The number of valence electrons in carbon atom is four. As such each carbon atom is linked with four neighboring carbon atoms. Thus forming a rigid tetrahedral structure. It is the strong bonding’that makes diamond the hardest substance.
(c) Fullerenes: Fullerenes are the third crystalline form of carbon.
Though they were discovered only recently. They have.been found to exist in interstellar dust as well as in the geological formations of the earth.
Common fullerenes are C – 32, C – 50, C – 70 and C – 76
(d) Black diamonds have copper oxide present in them as impurity.
(e) In a graphite molecule of each carbon atoms is linked with three neighboring carbon atoms. Thus forming a hexagonal arrangement of atoms. These hexagonal grouping of carbon atoms are arranged as layers or sheets piled one the top of other. The layers are held together by weak forces such that they can slide over one another. That is why graphite is soft.
Question 8.
Give two uses of (a) graphite (b) diamond.
Answer:
(a) Uses of graphite:
- For making the electrodes of electric furnaces.
- For making crucibles for melting metals due to its high melting points.
(b) Uses of Diamond:
- Diamond is used in jewellery as a gem
- It is used for cutting and drilling rocks, glass,
Question 9.
Write three differences between graphite and diamond.
Answer:
Difference between diamond and graphite.
Diamond
- Pure diamond is colourless and transparent.
- It is the hardest naturally occurring substance.
- It has high density i.e. 3.5 g/cm3
- It is bad conduct of electricity.
- It bums in air at 900°C to form carbon dioxide.
Graphite
- Graphite is greyish black opaque and shiny.
- It is soft and greasy to touch.
- It has low density i.e. 2.39 g / cm3
- It is good conductor of electricity.
- It bums in air at 700° C to form carbon dioxide.
Exercise – II Carbon and Its Compounds ICSE
Question 1.
Fill in the blanks:
(a) Charcoal is formed when charcoal is burnt in a limited supply of air.
(b) Coal is a amorphous form of carbon.
(c) Peat is the most inferior form of coal.
(d) Wood charcoal is a bad conductor of heat and electricity.
(e) lampblack is used in making black shoe polish.
Question 2.
Choose the correct alternative
(a) Anthracite is
(i) an inferior type of coal
(ii) a superior type of coal
(iii) a cheapest form of coal
(iv) none of above
Answer:
a superior type of coal
(b) Destructive distillation of coal yields
(i) coal tar
(ii) coal gas
(iii) coke
(iv) a superior type of coal
Answer:
a superior type of coal
(c) Lamp black is
(i) an amorphous form of carbon
(ii) a crystalline form of carbon
(iii) a pure form of carbon
(iv) a cluster of carbon atoms
Answer:
an amorphous form of carbon
(d) The process by which decayed plants slowly convert into coal is called.
(i) petrification
(ii) carbonisation
(ii) carbonification
(iv) fermentation
Answer:
carbonisation
(e) The purest form of the amorphous carbon is
(i) wood charcoal
(ii) sugar charcoal
(iii) bone charcoal
(iv) lampblack
Answer:
sugar charcoal
Question 3. Carbon and Its Compounds ICSE
Write ‘true’ or ‘false’ against the following statements:
(a) Charcoal is a good adsorbent. True
(b) Coke is obtained by destructive distillation of sugar. False
(c) Activated charcoal is a good conductor of electricity. False
(d) Wood charcoal is an important constituent of gun powder. True
(e) Coal gas is used in the preparation of artificial ferilizers. False.
Question 4.
Define the following:
(a) Carbonization
(b) Adsorption
(c) Bone black
Answer:
(a) Carbonization: The process of the slow conversion of vegetable matter into carbon-rich substances is called carbonization.
(b) Adsorption: Adsorption is the property due to which a substance absorbs gases, liquids and solids on its surface.
(c) Bone black: The Carbon content of bone charcoal is separated by treating the latter with hydrchloride acid, which dissolves the calcium phosphate. Carbon is then filtered out of the solution and in this form it is called bone black.
Question 5.
Name the following:
(a) Substances whose atoms or molecules are not arranged in a geometrical pattern. – Amorphous
(b) The best variety of coal. – Bituminous
(c) The purest form of amorphous carbon. – Anthracite
(d) An amorphous form of carbon that contains about 98% carbon. – Anthracite
(e) Mixture of carbon monoxide and hydrogen. – Water gas.
Question 7.
Answer the following questions:
(a) What is destructive distillation? What are the products formed due to the destructive distillation of coal?
(b) Why is wood charcoal used in water filters and gas masks?
(c) How is wood charcoal made locally? What other substances are formed in the process.
(d) How many carbon atoms are there in Buckminster fullerenes?
Answer:
(a) Destructive Distillation: When a substances is heated in the absence of air. The process is called destructive distillation.
Products formed are: Coke, Coal tar, Coal gas and ammonia solution
(b) Due to its high adsorbing capacity, wood charcoal is used as gas masks to adsorb harmful gases. Wood charcoal is porous, that is why it is used to filter water.
(c) Wood charcoal is prepared when wood is heated in a limited supply of air. Locally wood charcoal is prepared by piling logs of wood one above the other with a gap in the centre of the pile. The pile is covered with wet clay to prevent the entry of air. A few holes are left at the bottom of the pile. The wood is set on fire. After some time when fire dies out, wood charcoal is left behind. The other substances are -wood tar, pyroligneous acid and wood gas.
(d) 60 carbon atoms are arranged in spherical structure in Buck minster fullerences.
Question 7.
(a) Descirbe the formation of coal,
(b) Name four types of coal with percentage of carbon present in each, with uses.
Answer:
(a) Formation of coal:- The formation of coal took millions of years. Coal was formed by the bacterial decomposition of ancient vegetable matter hurried under successive layers of the earth. Under in action of high temperature and pressure, and in the abcence of air, the decayed vegetable matter converted into coal.
(b) Types of Coal:
- Peat: It is light brown in colour and contains only 50 – 60% carbon. It is the most inferior form of coal.
- Lignite: it contains more than 60% carbon. It is brown in colour and harder than peat.
- Bituminous: It has 90%, 80%, 70 – 75% carbon contents. Bituminous coal is the most common variety of coal and used as house hold
- coal.
- Anthracite: It is the purest variety of coal. Its carbon contents vary between 92 – 98%. It is hard, dense and black, difficultto ignite.
Uses of coal:
- Coal is used as both domestic and industrial fuel.
- It is used to prepare coke, coal gas and coal tar.
Question 9.
Name the products formed when:
(a) wood is burnt in the absence of air.
(b) bone is heated in the absence of air.
(c) diamond is burnt in air at 900°C.
(d) graphite is subjected to high pressure and 3000°C temperature.
Answer:
(a) Wood charcoal is formed when wood is burnt in limited supply of air.
(b) Bone charcaol, bone oil and organic compound pyridine.
(c) Carbon dioxide.
(d) Artificial diamond.
Question 10 Carbon and Its Compounds ICSE
Give two uses for each of the following:
(a) coal
(b) coke
(c) wood charcoal
(d) sugar charcoal
(e) bone charcoal
(f) lampblack
Answer:
(a) Uses of coal
- It is used as both a domestic and an industrial fuel.
- It is used to prepare coke, coal gas and coal tar.
(b) Uses of coke
- Coke is used as a smokeless fuel, in smelting furnaces.
- It is used in the manufacturing of water and producer gas.
(c) Uses of wood charcoal:
- Wood charcoal is used as a fuel.
- It is an important constituent of gun powder.
(d) Sugar charcoal:
- Sugar charcoal is mostly used as a reducing agent.
- It is used to decolourise coloured solutions.
(e) Bone charcoal:
- It is extensively used to decolourise cane-sugar in the process of manufacturing sugar.
- It is also used in the manufacture of large number of phosphorous compounds.
(f) Uses of lamp black:
- It is used in making black shoe polish.
- It is used in the manufacture of tyres and gun powder.
Question 11
Give balanced equations for the following chemical reactions:
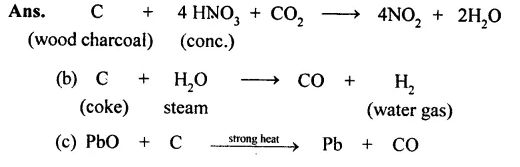
(a) wood charcoal and cone, nitric acid
(b) coke and steam
(c) wood charcoal and lead monoxide.
Exercise – III Carbon and Its Compounds ICSE
Question 1.
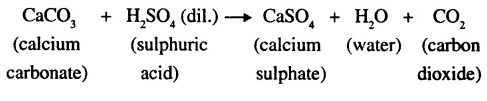
(a) Name the chemicals required for the preparation of carbon dioxide in the laboratory.
(b) How will you collect the gas ?
(c) Write the balanced chemical equation for the above reaction.
(d) Draw a labelled diagram for the preparation of CO2 in the laboratory.
(e) Why is sulphuric acid not used for the preparation of carbon dioxide in the laboratory ?
Answer:
(a) Calcium carbonate and dilute hydrochloric acid.
(b) By upward displacement of air.
(c) CaCO3 + 2HCl → CaCl2 + H2O + CO2.
(d) Laboratory preparation of carbon dioxide
(e) Dilute sulphuric acid reacts with calcium carbonate. But it is not used because the calcium sulphate which is formed during the reaction is insoluble in water. It covers the marble chips and stops the reaction.
Question 2. Carbon and Its Compounds ICSE
Write the balanced chemical equations for the preparation of carbon dioxide by:
(a) heating calcium carbonate.
(b) the action of acetic acid on sodium bicarbonate.
(c) the action of dilute sulphuric acid on sodium bicarbonate.
Answer:

Question 3.
What happens when:
(a) a lit splinter is introduced into a jar containing carbon dioxide ?
(b) moist blue litmus paper is placed in a jar containing carbon dioxide ?
(c) carbon dioxide is passed through lime water first in small amounts and then in excess ?
(d) a baking mixture containing baking powder is heated?
(e) a soda water bottle is opened ?
Answer:
(a) Lit splinter extinguishes.
(b) Blue litmus paper turns red.
(c) When CO2 is passed through lime water in small amount, it turns milky, when passed in excess milkiness disappears.
(d) Carbon dioxide is formed.
(e) When the pressure is released the bottled gas escapes with a bristling effervescence that ads fizz to the drink.
Question 4.
Give reasons for the following:
(a) An excess of carbon dioxide increases the temperature of the earth.
(b) Soda acid and foam types of fire extinguisher are not used for extinguishing electrical fires.
(c) Solid carbon dioxide is used for refrigeration of food.
Answer:
(a) Excess of carbon dioxide increases the temperature of the earth. Due to rise in temperature ice in the polar regions may melt causing floods in coastal regions island.
(b) In both of these fire extinguishers, the solutions are prepared in water, which conducts electricity. As a result an electric shock might result, which might lead to short- circuiting and another fire.
(c) Solid carbon dioxide serves as a coolant and refrigeration for preserving food articles.
Question 5.
What is a fire extinguisher ? What is the substance used in the modern type of fire extinguishers ? How is it an improvement over the soda acid-type and the foam-type fire extinguishers ?
Answer:
Fire Extinguisher— Fire extinguishers are a device in which carbon dioxide is produced in different forms for use as the extinguishing agent. It is a modem type of fire extinguisher in which liquid carbon dioxide is stored in a steel cylinder under pressure. Soda-acid and foam types of extinguisher cannot be used for extinguishing fire as they prepared in water, which conducts electricity and there can be short circuiting, causing another fire.
Question 6.
Explain the term ‘green house effect’. How can it be both beneficial and harmful for life on earth ?
Answer:
Green house effect— The trapping of the earth’s radiated energy by carbon dioxide present in air, so as to keep the earth warm, is called ‘green house effect’.
Green house is beneficial because this principle is applied to grow plants in colder regions.
Carbon dioxide increases the temperature of atmosphere. Due to rise in temperature; ice in the polar regions may melt, causing floods. So it is harmful for life on earth.
Question 7. Carbon and Its Compounds ICSE
What steps should be taken to balance carbon dioxide in the atmosphere ?
Answer:
As global warming will cause an unbalanced ecological system, serious efforts should be made to balance the percentage of carbon dioxide in the atmosphere. Some of these steps are:
- Growing more trees and plants.
- Using smokeless sources of energy like solar energy, biogas, etc.
- Using filters in the chimneys of factories and power houses.
Question 8.
State three ways by which carbon dioxide gas is added into the atmosphere.
Answer:
- planting more trees.
- combustion of fuels
- decay of dead animals, plants and plants products.
Exercise – IV Carbon and Its Compounds ICSE
Question 1.
Fill in the blanks:
(a) Carbon monoxide is formed when carbon is burnt in a limited supply of air or oxygen.
(b) Carbon monoxide bums in air with a pale blue flame to form carbon dioxide.
(c) Carbon monoxide is a products of incomplete combustion.
(d) A mixture of 95% oxygen and 5% carbon dioxide is called carbogen
(e) Carbon dioxide is used as a reducing agent in the extraction of pure metals from their corresponding ores.
Question 2.
Match the following.

Question 3.
How is carbon monoxide gas formed?
Answer:
Mostly carbon monoxide is formed when a large amount of carbon or its compounds is burnt in a limited supply of air or oxygen.
Question 4.
State the poisonous nature of carbon monoxide?
Answer:
Carbon monoxide is highly poisonous gas. If air containing 0.5% carbon monoxide by volume is inhaled, death can result This is because carbon monoxide combines with the haemogloblin present in the blood cells of our body to form a stable compound called carboxyl-haemoglobin. This does not allow to absorb oxygen. Thus depriving our body cells of oxygen. This cause obsruction in respiration and causes death.
Question 5. Carbon and Its Compounds ICSE
Give two uses of carbon monoxide.
Answer:
Uses of carbon monoxide:
- Carbon monoxide is a strong reducing agent.
- Carbon monoxide is used in the extraction of pure metals from their ores.
Question 6.
Why is carbon monoxide called silent killer ?
Answer:
Carbon monoxide is produced by burning coal or wood in a limited supply of air. Since the gas is colourless and a barely detectable smell, people do not feel it and it can be proved as a silent killer.
Question 7.
Explain the reducing action of carbon monoxide.
Answer:
Reducing action of carbon- monoxide: Carbon monoxide is a strong reducing agent. It reduces the oxides of the less active metals to their respective metals and itself gets oxidised to carbon dioxide.
Question 8.
Write two remedies for carbon monoxide poisoning.
Answer:
- The victim should immediately brought out into the open.
- The victim should be given artificial respiration with caibogen.
Question 9.
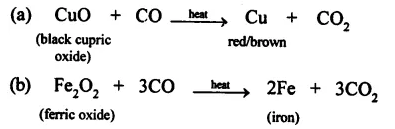
Complete the reactions and balance them.
(a) CuO + CO →
(b) Fe2O2 + CO →
Answer:
Return To ICSE Class-8 Concise Selina Physics Solution
Thanks