Chemical Bonding Dalal Simplified ICSE Chemistry Class-10 Solutions Chapter 2 . Solutions of Dr Dalal Simplified ICSE Chemistry Chemical Bonding by Dr Viraf and J Dalal for Class 10. Step by step Solutions of Chemical Bonding ICSE Chemistry by Dr Viraf and J Dalal
Get Other Chapter Dalal Simplified ICSE Chemistry Class-10 Solutions
How to Solve Chemical Bonding ICSE Chemistry Class-10
Note:– Before viewing Solutions of Chemical Bonding by Dr Viraf and J Dalal Simplified ICSE Chemistry Solutions of Chapter-2.Read the Chapter-2 Chemical Bonding Carefully to understand the concept in better way .After reading the Chapter-2 Chemical Bonding solve all example of your text book with ICSE Specimen Sample Paper for Class-10 Exam of Council. Focus on Numerical’s Problems. Chemical Bonding is the Most important Chapter in ICSE Class 10 Chemistry.Previous Year Solved Question Paper for ICSE Board
Additional Questions Chemical Bonding Dalal Simplified
Question 1.
State the force which holds two or more atoms together as a stable molecule.
Answer:
Chemical bond.
Question 2.
Draw the geometrical atomic structure representing the electronic configuration of atoms of elements of
(a) Period-2
- group 14 (IV A)- carbon (at no. 6)
- group 15 (VA) – nitrogen (at. no. 7)
- group 16 (VI A) – oxygen (at no. 8)
(b) Period-3
- group 1(IA) – sodium (at no. 11)
- group 2(HA) – magnesium (at. no. 12)
- group 17(VDLA) – chlorine (at. no. 17)
(c) Period -4
- group 2(ELA) – calcium (at. no. 20).
Answer:
(a)
(a)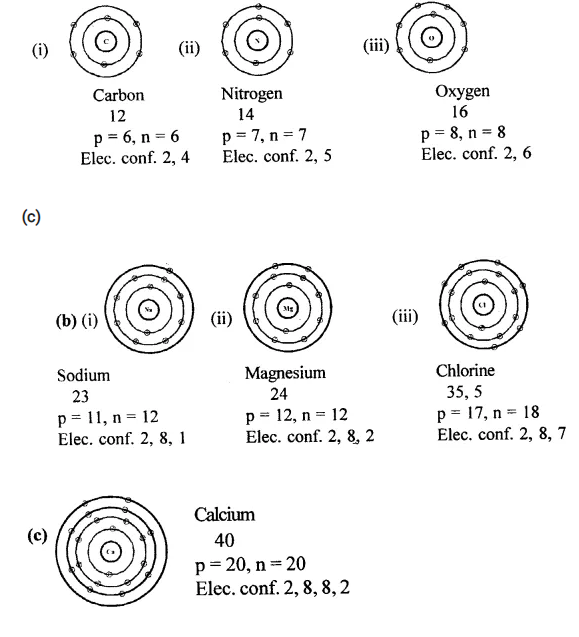
Question 3.
What is meant by the term ‘chemical bond’ and ‘chemical bonding’.
Answer:
Chemical bond:
The linkage or force which acts between two or more atoms to hold them together as a stable molecule is called a chemical bond.
Chemical bonding:
The concept of chemical bond is called chemical bonding.
Question 4.
State why noble gases are unreactive while atoms of elements other than noble gases are chemically reactive.
Answer:
Because they have stable electronic configuration that is their outermost shell is complete.
Question 5.
State the reasons for chemical bonding between two atoms and the methods involved for achieving the same. State how ‘duplet and octet’ rules are involved for an atom to achieve stable electronic config.
Answer:
The driving force for atoms to combine is related to the tendency of each atom to attain stable electronic configuration of nearest noble gas. For an atom to achieve stable electronic configuration it must have;Either two electrons in its outermost shell (if it is the first shell- nearest noble gas – He) – Duplet rule.
OR
Eight electrons in its outermost shell (if it is not the first shell – all noble gases other than He have eight electrons in their outermost shell) – Octet rule.
Methods for achieving chemical bonding
A stable electronic configuration for two combining atoms, resulting in chemical bonding between them is achieved by following two ways.
- Electron transfer: This involves transference of valence electrons from one atom (metal) to another (non-metal) leading to the formation of electrovalent or ionic bond. This results in the formation of electrovalent or ionic compound.
- Electron sharing: This involves sharing of pairs of electrons between two atoms (both non-metals). This leads to the formation of covalent bond. The compound so formed is called a covalent compound.
Question 6.
State the type of compounds formed by transfer of valence electrons from one atom to another, and explain the method of formation of the same. State the role of ‘cations’ and ‘anions’ in their formation.
Answer:
The type of compounds formed by transfer of valence electrons from one atom to another is ionic or Electrovalent compounds. The atom which loses electron is generally a metallic atom while the atom which gain electrons is generally a non-metallic atom.
The metallic atom loses electrons to attain stable electronic configuration and becomes a cation. For example,
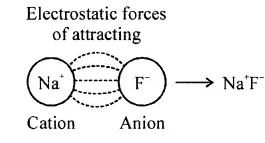
The non-metallic atom gains electron to attain stable electronic configuration and becomes an anion. For example,
Na Na
The cations and anions so formed are oppositely charged ions, which attract one another to from an electrovalent or ionic bond. This results in the formation of electrovalent or ionic compound (NaF).
………………
The bond formation is due to the electrostatic forces of attraction between two oppositely charged ions.
Question 7.
Define the terms:
- Electrovalent or ionic bond
- Electrovalent or ionic compound.
Answer:
- Electrovalent or ionic bond: The chemical bond formed between two atoms by transfer of one or more electrons from the atom.
- Electrovalent or ionic compound: The chemical compound formed as a result of transfer of one or more electrons from atom.
Question 8.
What is meant by the term ‘electrovalency’. State why Na (at. no. 11) has a electropositive valency of +1 and Cl (at. no. 17) an electronegative valency of -1.
Answer:
Electrovalency: The number of electrons donated or accepted by the valence shell of an atom of an element so as to achieve . the stable electronic configuration is called electrovalency, stable configuration of nearest noble gas i.e., Ne (Z = 10)
…………………
Na → Na+ + e
(2,8,1) (2,8)
As sodium (Z = 11) loses one electron to achieve it has an electrovalency of + 1
Cl + e- → Cl-
(2,8,7) (2,8,8)
As chlorine (Z = 17) gains one electron to achieve stable configuration of nearest noble gas i.e., Ar (Z = 18) it has an electrovalency of – 1.
Question 9.
State three differences between ‘X’ and ‘X1+’ i.e. an atom and an ion.
Answer:
Atoms – ‘X’
- Electrically – neutral particles
- May or may not exist – independently
- Outermost shell – may or may not have duplet or octet.
Ions – X1+
- Electrically-charged particles [cations, anions]
- Exist – independently in solution
- Outermost shell -have complete duplet or octet.
Question 10.
Explain the terms ‘oxidation’ and reduction’ with reference to an atom or ion.
Answer:
oxidation: Loss of one or more electrons by an atom or ion is called oxidation.
For example,
Na → Na+ + e–
Fe2+ → Fe3+ + e-
Mg → Mg2+ + e-
Reduction: Gain of one or more electrons by an atom or ion is called reduction.
For example,
F + e– → F–
O + 2e- → O2–
Cu2+ + e– → Cu+
Zn2 + 2e– → Zn
Question 11.
State which of the following are oxidation reactions and which are reduction reactions.
(a)
- Cu → Cu2+ + 2e–
- Cu2+ + 2e– → Cu
- Sn4+ + 2e–→Sn2+
- 2Cl- → Cl2 + 2e-
- Fe2+ → Fe3++le–
- X +2e–→X2-
- Y-le– → Y1+
- Z3++le–→Z2+
Answer:
Oxidation reactions:
(3) Sn4+ + 2e–→Sn2+
(6) X +2e–→X2-
(7) Y-le– → Y1+
(8) Z3++le–→Z2+
Reduction reactions.
(1) Cu → Cu2+ + 2e–
(2) Cu2+ + 2e– → Cu
(4) 2Cl- → Cl2 + 2e-
(5) Fe2+ → Fe3++le–
(b)
- Zn → Zn2+
- S → S2-
- Sn2+→ Sn
- Fe 2+→ Fe3+
Answer:
(1) Oxidation (2) Reduction (3) Reduction (4) Oxidation
Question 12.
Explain with the help of
(1) An ionic equation
(2) Electron dot structural diagram
(3) Atomic or orbital structural diagram the formation of the following.
(a) Sodium chloride
(b) Calcium oxide
(c) Magnesium chloride.
(at. nos. Na = 11, Cl = 17, Ca = 20, O = 8, Mg = 12)
Answer:
(a) Formation of Sodium Chloride
(1) Ionic equation
………………
(2) Electron dot structure
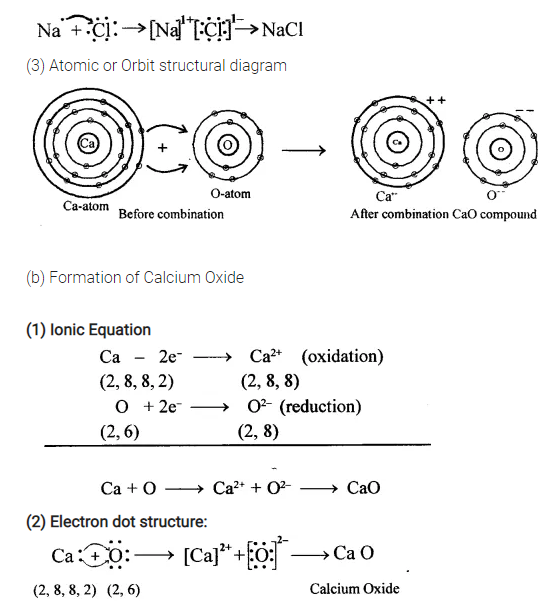
(3) Atomic Or Orbit Structural Diagram
…………..
……………………
Fill in the blanks with the appropriate word /s from the brackets.
Question 13
(i).
NaCI an electrovalent compounds is formed as a result of transfer of— (one, two, three) valence electrons from metallic sodium to non-metallic chlorine atom. CaO is similarly formed as a result of transfer of…………… (1,2,3) valence electron/s from metallic calcium to non-metallic oxygen and magnesium chloride by transfer of–(one, two, three) valence electron/s from ………..(one, two) magnesium atom/s to……….. (one, two) chlorine atom/s.
Answer:
NaCI an electrovalent compounds is formed as a result of transfer of one valence electrons from metallic sodium to non- metallic chlorine atom. CaO is similarly formed as a result of transfer of two valence electron/s from metallic calcium to non-metallic oxygen and magnesium chloride by transfer of two valence electron/s from one magnesium atom/s to two chlorine atom/s.
Question 13(i).
Covalent compounds are formed by sharing electron pairs between non-metallic atoms. Non-metallic atoms having….,……. valence electrons (4, 5, 6, 7) share one, two or three pairs of electrons respectively.
Answer:
Covalent compounds are formed by sharing electron pairs between non-metallic atoms. Non-metallic atoms having 7,6, 5 valence electrons (4, 5, 6, 7) share one, two or three pairs of electrons respectively.
Question 14.
Define or explain the terms:
- covalent or molecular bond
- covalent or molecular compound
- Covalency
- Shared pair of electrons.
Answer:
(1) Covalent bond: The chemical bond formed due to mutual sharing of electrons between the given pairs of atoms of non-metallic elements. In the bond formed by a shared pair of electrons, each bonding atom contributing one electron to the pair. Depending on number of electron pairs shared bond is single [-], double [=], or triple [ = ] covalent.
(2) Covalent compound: The chemical compound formed due to mutual sharing of electrons between the given pairs of atoms forming a covalent bond is called covalent compound.
(3) Covalency: The number of electron pairs which an atom shares with one or more atoms of the same or different kind to achieve stable electronic configuration is called covalency.
(4) Shared pair of electrons: A pair of electrons which is shared between two atoms resulting the formation of a covalent bond is called a shared pair of electrons.
Question 15.
Give two differences between the covalent compounds – methane (non-polar) and HCl (polar)
Answer:
Non-polar covalent compound(Methane,CH4)
- Covalent compounds are said to be non-polar when shared pair of electrons are equally distributed between the two atoms.
- No charge separation takes place.The covalent molecule is symmetrical and electrically neutral.
………………
Polar covalent compound (HCl)
- Covalent compounds are said to be polar when shared pair of electrons are unequally distributed between the two atoms.
- Charge separation takes place.The atom which attracts electrons more strongly develops a slight negative charge while the other develops a slight positive charge.
………..
Question 16.
Explain with the help of –
(1) electron dot diagram
(2) atomic or orbital structural diagram – the formation of the following molecules, stating the valency of each element involved.
(a) Hydrogen
(b) Chlorine
(c) Oxygen
(d) Nitrogen
(e) Water
(f) Methane
(g) Carbon tetrachloride
(h) Ammonia
(i) Carbon dioxide
(at. nos. H = 1, C = 6, N = 7, O = 8, Cl = 17)
Answer:
(a) Formation of Hydrogen molecule (H2) (Non-polar covalent compound).
Atom 11H – it needs one electron to achieve a stable configuration
- Electron Dot Structure
………………….
- Atomic or orbit structural diagram
………
(b) Formation of Chlorine (Cl2) (non-polar covalent compound)
Chlorine atom Cl electronic configuration (2,8,7)— It needs one electron to attain stable configuration
- Electron Dot Structure
………….
- Atomic or orbit structural diagram
…………
(c) Formation of Oxygen molecule (O2)
Oxygen atom 816O electronic configuration 2,6
When two oxygen atoms contributes two electrons so as to have two shared pair of electrons between them thereby both atoms attain stable octet structure resulting in the formation of double bond [O = O] between them.
- Electron Dot Structure
……. - Atomic or orbit structural diagram
………
(d) Formation of Nitrogen Molecule (N2) (non-polar covalent compound)
Nitrogen electronic configuration (2,5). When two nitrogen atoms come close, each contributes three electrons to share to attain stable octet structure resulting in formation of triple bond (N = N) – N2.
- Electron Dot Structure
………….
Atomic or orbit structural diagram
……
(e) Formation of Water molecule (H20)
Hydrogen 11H electronic structure 1.
Oxygen 816O electronic structure 6 needs two electrons to complete octet.
When a molecule of water is formed, each of two hydrogen atoms share one electron pair with oxygen atom.
- Electron Dot Structure
………..
- Atomic or orbit structural diagram
……………
(f) Formation of Methane moleucle (CH4)
Carbon atom 12 6C electronic configuration (2,4)
Hydrogen 11H
When a molecule of methane is formed one atom of C shares four electron pairs, one with each of four atoms of Hydrogen
- Electron Dot Structure
….
- Atomic or orbit structural diagram
…………
(g) Formation of Carbon Tetrachloride (CCl4)
Carbon 12 6C (2,4) Carbon needs- four electrons to attain – stable octet.
Chlorine 17 35Cl (2,8,7) Chlorine needs – one electron to attain – stable octet.
- Electron Dot Structure
………..
- Atomic Or orbit Structural Diagram
……..
(h) Formation of Ammonia molecule (NH3)
Electronic configuration of H (Z = 1) and N (Z = 7) are
K L
H(Z= 1): 1,
N(Z=7):2 5
Hydrogen has one electron in its outermost shell and nitrogen has five electrons in its outermost shell.
- Electron dot diagram
……… - Atomic or orbital structural diagram
…….
(i) Atomic or orbital structural diagram
Carbon – C (2,4) Carbon needs- four electrons to attain – stable octet.
Oxygen O (2, 6) Oxygen needs – two electron to attain – stable octet.
- Electron Dot Structure
………..
- Atomic or orbit structural diagram
…..
Question 17 (i).
Give reasons for the following:Molecules of hydrogen and chlorine have single covalent bonds between their atoms while oxygen has a double covalent and nitrogen a triple covalent bond respectively.
Answer:
Hydrogen – Each of the two H atoms contributes one electron so as to have one shared pair of electrons between them.Both atoms attain stable – duplet structure, resulting in the – formation of a -single covalent bond [H-H] between them.
……
Chlorine – Each of the two Cl atoms contributes one electron so as to have one shared pair of electrons between them. Both atoms attain stable – octet structure, resulting in the – formation of a – single covalent bond [Cl – Cl] between them.
……..
Oxygen – Each of the two O atoms contributes two electron so as to have two shared pair of electrons between them. Both atoms attain stable – octet structure, resulting in the – formation of a – double covalent bond [O = O] between them
……………………
Nitrogen- Each of the two N atoms contributes three electron so as to have three shared pair of electrons between them. Both atoms attain stable – octet structure, resulting in the – formation of a – tripple covalent bond [N = N] between them.
……
Question 17 (ii)
A molecule of methane has four single covalent bonds.
Answer:
Methane – Non – polar covalent compound
….
When a molecule of ‘CH.’ is to be formed –
One atom of carbon thus – shares four electron pairs – one with each of the four atoms of hydrogen.
Formation of Methane Molecule can be represented by
Atomic or orbit structural diagram
…………
Question 17 (iii)
Formation of ammonia involves one atom of nitrogen sharing three electron pairs one with each of the three atoms of hydrogen.
Answer:
Ammonia – Polar covalent compound
…..
When a molecule of ‘NH3’ is to be formed – One atom of nitrogen shares three electron pairs one with each of the three atoms of hydrogen.
Formation of Ammonia Molecule can be represented by Atomic or orbit structural diagram
….
Question 18.
Explain the terms
(a) Lone pair of electrons.
Ans. Lone pair of Electrons – are a pair of electrons not shared with any other atom.
(b) Coordinate bond. Explain diagrammatically the lone pair effect of:
(1) The nitrogen atom of the ammonia molecule leading to the formation of ammonium ions (NH4)+
Answer:
Coordinate Bond: It is a type of covalency which involves one of the combining atoms contributing both of the shared electrons, i.e. a bond formed by a shared pair of electrons with both electrons coming from the same atom.
Formation of Ammonium Ion – NH4+
………….
(2)
The oxygen atom of the H20 molecule leading to formation of hydronium (H2O)+ and hydroxyl ions (OH)–
Ans.
Formation of Hydronium Ion [H30]+ and hydroxyl [OH]– Ions
Question 19.
Give reasons for the following:
Electrovalent compounds are soluble in water, insoluble in organic solvents, good conductors of electricity in molten or aq. solution state, have high melting points and undergo electrolytic dissociation on passage of electric current, while covalent compounds are soluble in organic solvents, insoluble in water, non-conductors of electricity, have low melting points and undergo ionisation on passage of electric current.
Answer:
1 Solubility — *Soluble – in water, * Insoluble – in organic solvents.
Reason: Water [polar solvent] has a high dielectric constant i.e. capacity to weaken the force of attraction, thus resulting in free ions. Organic solvents [non-polar] have low dielectric constants and do not cause dissolution.
2 Conduction of Electricity —*Solid state –
Non-conductors, *Molten or aq. soln. state – Good conductors
Reason: Strong electrostatic force keeps ions in fixed position . in the – solid state.
The force is weakened in the molten state and disappears in soln. state, hence free ions formed migrate to – oppositely charged electrodes.
3 Melting and Boiling Point — *
High melting point and high boiling point.
Reason: Strong electrostatic force of attraction between ions. Large amount of energy – required to break the force of attraction.
4 Electrolysis
— * Can – be electrolysed in molten/aq. soln. state. Reason on electrolysis the ions being charged are attracted towards the respective electrodes.
5 Dissociation
— * Undergoes electrolytic dissociation – on passage of electric current. Process involves – separation of ions already present in the ionic compound.
……………..
Properties of covalent compounds:
1 Covalent compounds are soluble in organic solvents but insoluble in water.
Reason: Organic solvents the benzene, carbon tetrachloride, hexane are non-polar in nature. Non-polar solvents dissolve non-polar covalent compounds (like1 dissolves like). Thus, water – a polar solvent cannot dissolve non-polar compounds.
2 Covalent compounds are non-conudctors of electricity.
Reason: Non-polar compounds (like CCl4) contain molecules and not ions. Hence non-polar covalent compounds do not conduct electricity. Polar covalent compounds (like HCl) when dissolved in water produce ions and hence conduct electricity.
3 Covalent compounds have low melting points.
Reason: The intermolecular forces of attraction in covalent compounds are weak van der Waals forces. Thus less amount of energy is required to break these forces of attraction resulting in a lower melting point.
4 Polar covalent compounds undergo ionisation when dissolved in water.
Such a solution can be electrolysed by passing electricity through it.
Reason: Polar covalent compounds (like HCl) dissolve in polar solvents like water (like dissolves like). This results in ionisation.
………………..
When electricity is passed through such a solution, ions migrate towards oppositely charged electrodes and are discharged.
2H+ (aq) + 2e– → H2 (g) At cathode
2Cl –(aq) → Cl, (g) + 2e At anode
–Try Also :–



Answer are long with mistakes
please send image of latest editions exercise question in sequence on my WhatsApp number 8948221203 followed by ch-name writer name class and page number