Chemistry Specimen 2024: Sec-B ISC Sample Model Paper Solved by expert teachers. Sample paper gives a blue print of upcoming exam. Therefore we have solved it according council latest guideline for 2024 exam. Visit official website CISCE for detail information about ISC Board Class-12.

Chemistry Specimen 2024: Sec-B ISC Sample Model Paper Solved
| Board | ISC |
| Class | 12th (xii) |
| Subject | Chemistry (Section-B) |
| Topic | ISC Specimen Paper Solved |
| Syllabus | on latest syllabus |
| Session | 2023-24 |
ISC Class-12 Chemistry Specimen Paper 2024 Solved Sec-B
Question 2 (i) Arrange the following alcohols in order of decreasing activity towards Lucas’ reagent.
2-butanol, 2-methyl-2-propanol and 1-butanol
Ans: the correct sequence of reactivity is : 2-methyl-2-propanol (3∘)>2-butanol (2∘)gt1-butanol (1∘
(ii) Ethanol has a higher boiling point than methoxymethane. Justify the statement
Ans: Because of the presence of the –OH group in ethanol, intermolecular H–bonding occurs, resulting in molecule interaction. To break these hydrogen bonds, more energy is required. Methoxymethane, on the other hand, does not undergo hydrogen bonding. As a result, ethanol has a greater boiling point than methoxymethane
Question 3 Give a reason for each of the following:
(i) The size of the trivalent cations in Lanthanoid series decreases steadily as the atomic number increases.
Ans: The atomic size or the ionic radii of tri-positive lanthanide ions decrease steadily from La to Lu due to increasing nuclear charge and electrons entering the inner (n-2) f orbital. This gradual decrease in size with an increasing atomic number is called lanthanide contraction
(ii) The third ionization energy of manganese (Z = 25) is unexpectedly high
Ans : Loss of two electrons gives the configuration of Mn2+ as; [Ar]3d5 where d-orbital is half filled and thus, very stable
Question 4 Give balanced chemical equations to convert the following:
(i) Benzene to biphenyl
(ii) Propene to propane -1-ol
Ans: (i) Make Chlorobenzene by reaction of Cl2 + FeCl3 with benzene. The Fittig reaction of Chlorobenzene will lead to Biphenyl as product. Fittig reaction is same as Wurtz but we take aryl halide as substrate. In this reaction, the substrate which is aryl halide, is reacted with Na in ether so the coupling of two molecules of aryl halide occurs eliminating halogen atoms.
C6H6 + Cl2———→C6H5Cl + HCl
2C6H5Cl +2Na——→C12H10(Biphenyl) + 2NaCl
(ii) Step 1:Formation of 1-Bromo Propane At first, Propene reacts with Hydrogen bromide in the presence of Peroxide to form 1-Bromo Propane.
Step 2:Formation of Propan-1-ol: The produced Alkyl halide reacts with KOH followed by heating produces Propan-1-ol as the final product.
Question 5 Account for the following:
(i) Salts of cuprous (Cu+) ion are colourless whereas the salts of cupric (Cu2+) ion are coloured.
Ans: Cu+ ion has an electronic configuration 3d10 whereas Cu2+ ion has an electronic configuration 3d9. Hence an unpaired electron is present in Cu2+ which makes electronic transition feasible in case of Cu2+ ion, thus imparting color. Due to lack of unpaired electrons, Cu+ is colorless
(ii) Zinc is not regarded as a transition element. (at. no. of Zn = 30)
Ans: Zinc is not a transition metal because it forms only Zn2+ ions with all the 3d electrons present. Because Zn has 3d orbital fullfil by 10 electrons, they are paired. that’s why they can’t release this electrons only lose ns electron
Question 6 Two compounds, D-2-chlorobutane and L-2-chlorobutane, are enantiomers of each other. Name one physical property that is:
(i) same for D-2-chlorobutane and L-2-chlorobutane.
Ans: 2-Chlorobutane has one chiral carbon atom and thus, exists as a pair of enantiomers which are non-superimposable mirror images of each other.
(ii) different for D-2-chlorobutane and L-2-chlorobutane
Ans: differ from each other in terms of a measurable property called optical activity.
Question 7 (i) A rusted piece of iron undergoes electrochemical reactions. Write the chemical reaction taking place at:
(a) the electrode that behaves as an anode.
(b) the electrode that behaves as a cathode.
Ans :- At anode, Fe is oxidized to Fe(II) ions and at cathode oxygen is reduced to water. The overall reaction is 2Fe(s)+O2(g)+4H+(aq)→2Fe2+(aq)+2H2O(l)
Fe(II) ions are further oxidized to Fe(III) ions. 4Fe2+(aq)+O2(g)+4H+(aq)→4Fe3+(aq)+2H2O(l)
Fe(III) ions form an insoluble hydrated oxide which is deposited as red brown material called rust. 2Fe3+(aq)+4H2O(l)→Fe2O3H2O(s)+6H+(aq)
reaction at cathode : The reaction at the cathode: O2(g) + 4H+ (aq) + 4e − → 2H2O(l) E° = 1.23V. In this reaction, the Oxygen combines with hydrogen ions and forms water which causes rusting on the surface of iron meta
(ii) Given that the standard reduction potential for Al3+/Al = -1ˑ66 V and ½ I/I- = 0ˑ54V, what will be the standard potential of the cell made by using Al3+ and I- ?
Ans : E cell = E reduced – E oxidised = + 0.54 V – (-1.66 V) = + 2.2 V
Question 8 (i) What happens when (write chemical reactions only) (
a) Diethyl ether is treated with phosphorous pentachloride.
Ans :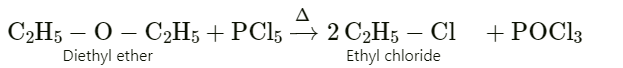
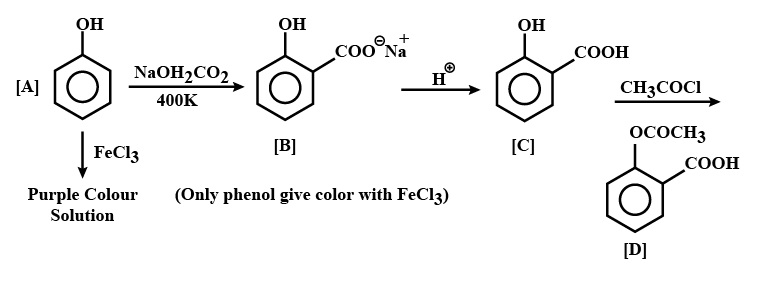
(b) Ethyl alcohol is treated with methyl magnesium bromide (ii) An organic compound [A] having molecular formula C6H6O gives a characteristic colour with aqueous FeCl3 solution. [A] on treatment with CO2 and NaOH at 400K under pressure gives [B] which on acidification gives compound [C]. [C] reacts with acetyl chloride to give [D] which is a popular pain killer. Identify the compounds [A], [B], [C] and [D].
Ans:
Question 9 John was making noodles in boiling water. When he added common salt (NaCl) to boiling water, the water stopped boiling for a short while. If John had added 15ˑ0g of NaCl to 250ˑ0g of water, calculate the boiling point of solution assuming that NaCl dissociates completely in water. (Kb for water = 0ˑ512K kg mol-1 , molecular mass of NaCl = 58ˑ44 g mol-1 )
Ans :– mass of solute = 15 g, mass of solvent = 250 g Kb= 0.512, Molecular mass of NaCl = 58.44 and i = 2,
ΔTb =( i×Kb×1000 x w ) / (W x mm)
= (2 x 0.512 x 15 x 1000 ) / (250 x 58.44)
=1.05 , So Boiling Point = 100 + 1.05 = 101.5 C.
Question 10 (i) Aromatic aldehydes do not give a reddish-brown precipitate on heating with Fehling solution. Give a reason.
Ans : In aromatic aldehydes, the CHO group is attached to a benzene ring. Due to resonance, carbonyl group’s C acquires a double bond character with the benzene which is very strong to break. oxidizing agents like Cu2+ are unable to break that bond, so such aldehydes are unable to show fehling’s test.
(ii) Why is benzaldehyde less reactive to electrophilic substitution reactions than benzene?
Ans : Benzaldehyde is less reactive than benzene since the carbonyl of the aldehyde group is electron attracting and hence decreases the electron density in benzene ring
Question 11 (i) Give a reason to explain why transition metals can act as a good catalyst.
Ans :- Transition metals have partially filled d- orbitals so they can easily withdraw the electrons from the reagents or give electrons to them depending on the nature of the reaction. They also have a tendency to show large no. of oxidation states and the ability to form complexes which makes them a good catalyst
(ii) Scandium (Z = 21) does not exhibit variable oxidation states and yet it is regarded as transition element. Why?
Ans :– Scandium ( ) does not exhibit variable oxidation states and yet it is regarded as a transition element because scandium has partially filled -orbitals in the ground state ( 3 d 1 4 s 2 )
— : end of Chemistry Specimen 2024: Sec-B ISC Sample Model Paper Solved : —
–: Visit also :–
Return to : ISC Specimen Paper 2024 Solved
Thanks