Chemistry Specimen 2024: Sec-C ISC Sample Model Paper Solved by expert teachers. Sample paper gives a blue print of upcoming exam. Therefore we have solved it according council latest guideline for 2024 exam. Visit official website CISCE for detail information about ISC Board Class-12.

Chemistry Specimen 2024: Sec-C ISC Sample Model Paper Solved
| Board | ISC |
| Class | 12th (xii) |
| Subject | Chemistry (Section-C) |
| Topic | ISC Specimen Paper Solved |
| Syllabus | on latest syllabus |
| Session | 2023-24 |
SECTION – C
ISC Class-12 Chemistry Specimen Paper 2024 Solved
Question 12 The data in the table given below was obtained in a series of experiments on the rate of the reaction between compounds [A] and [B] at a constant temperature

Show how this data can be used to deduce the rate expression for the reaction between [A] and [B].
Ans: 
Question 13 Arrange the following compounds: C6H5NH2, (C2H5)2NH, (C2H5)3N, C2H5NH2.
(i) in the increasing order of their basic strength in water.
(ii) in a decreasing order of their basic strength in gas phase.
Ans: (i) Increasing order of basic strength:- (C2H5)2NH>(C2H5)3N>C2H5NH2>C6H5NH2
(ii) decreasing order of their basic strength in gas phase (C2H5)3N > (C2H5)2NH > C2H5NH2 > C6H5NH2
Question 14
(i) What products are obtained when sucrose is subjected to acid hydrolysis?
(ii) Why are Vitamin B and Vitamin C essential for us?
(iii) On being heated, egg white becomes solid and opaque. Give a reason:
Ans: (i) The hydrolysis of sucrose generates an equimolar mixture of fructose and glucose, commercially known as invert sugar (ii) itamins B and C are two of the most important. Not only do they support a strong immune system and help your body to ward off illness, but they can also help your body to function optimally and give you energy (iii) An egg white is rich in protein. When cooked, it becomes firm and opaque. This is because the heat of cooking denatures the protein, causing it to unfold. As the proteins unfold, their hydrophobic sections become exposed and the proteins begin to form a white, solid meshwork.
Question 15 Water vapor and liquid water are in equilibrium in a container. At room temperature, the vapor pressure of water is 25 mm of Hg. The volume of water is V ml.
(i) What will be the vapor pressure of water if the volume of water is reduced to V/4 ml without any change in temperature? Give a reason.
(ii) Will there be a change in vapors pressure if more water (at room temperature) is added to the container? Give a reason.
Ans: (i) If the volume of water is reduced to v/4 ml without any change in temperature, the vapor pressure of water will remain the same. This is because the vapor pressure of a substance only depends on its temperature, not its volume (ii) No
Question 16 Identify the compounds [A], [B] and [C].
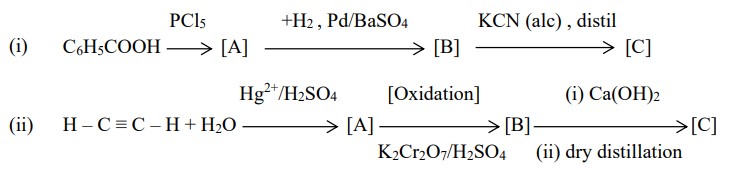
Ans :- (i) Benzoic acid reacts with PCl5 to form benzoyl chloride. Reduction with H2−Pd/BaSO4 gives benzaldehdye. Reaction with alcoholic KCN gives cyanohydrin
(ii) Hydration of ethyne with H2O/HgSO4/H2SO4 gives acetaldehyde. Hydrogenation in presence of Ni catalyst gives ethanol. On heating with conc sulphuric acid at 140oC, diethyl ether is obtained.
Question 17 (i) How will the following be obtained? (Give chemical equation)
(a) Picric acid from Phenol-–Picric acid is basically prepared from phenol by reacting phenol with concentrated sulphuric acid and concentrated nitric acid. The nitro groups from nitric acid attacks on ortho and para positions which is the best suitable and stable position for the newly formed compound which is picric acid
(b) Ethyl acetate from ethanol-— Ethyl acetate can be distilled by redistilling a mixture of ethanol and water, followed by condensation of water vapor with sodium acetate solution.
(c) Anisole from sodium phenoxide—Anisole is prepared by the action of methyl iodide on sodium phenoxide. This reaction is called Williamson’s synthesis. Williamson’s synthesis: This is one of the best methods for the preparation of ethers. It involves the treatment of an alkyl halide with a suitable sodium alkoxide
or
(ii) Explain the mechanism of acid catalyzed dehydration of ethanol to yield the corresponding alkene.
Ans:– Dehydration of ethanol takes place in presence of an acid because acid provides a proton so that oxygen from the ethanol can be attracted towards it and then the proton gets attached to it to form oxonium ion. This is the first step in the mechanism of acid dehydration of ethanol
Question 18
(i) The half-life period (t ½) for decay of radioactive 14C is 5730 years. An ancient piece of wood has only 80% of the 14C found in a living tree. Calculate the age of the piece of wood.
Ans:-
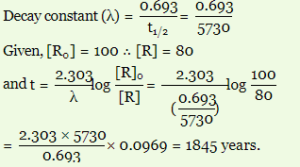
(ii) The rate of most of the reactions becomes double when the temperature is raised from 298K to 308K. Calculate the activation energy. (R = 8ˑ314 J K-1 mol-1 )
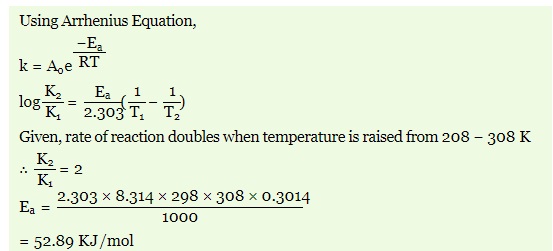
— : end of Chemistry Specimen 2024: Sec-C ISC Sample Model Paper Solved : —
–: Visit also :–
Return to : ISC Specimen Paper 2024 Solved
Thanks