Chemistry Specimen 2024: Sec-D ISC Sample Model Paper Solved by expert teachers. Sample paper gives a blue print of upcoming exam. Therefore we have solved it according council latest guideline for 2024 exam. Visit official website CISCE for detail information about ISC Board Class-12.

Chemistry Specimen 2024: Sec-D ISC Sample Model Paper Solved
| Board | ISC |
| Class | 12th (xii) |
| Subject | Chemistry (Section-D) |
| Topic | ISC Specimen Paper Solved |
| Syllabus | on latest syllabus |
| Session | 2023-24 |
SECTION – D ISC Class-12 Chemistry Specimen Paper 2024 Solved
Question 19
(i) Give a reason for each of the following:
(a) Formaldehyde does not undergo aldol condensation, but acetaldehyde does.— Carbonyl compounds containing alpha hydrogen atom undergoes aldol condensation reaction. Formaldehyde HCHO does not contain alpha hydrogen atom. Hence, it does not undergo aldol condensation reaction
(b) Chloroacetic acid is stronger acid than acetic acid.—- Chloroacetic acid is stronger than acetic acid because of the electron-withdrawing effect of chlorine. This effect is caused by the electronegativity of chlorine. The withdrawing effect means the negative charge carried on the acetate anion is spread more widely on the molecule, which stabilizes the acetate ions.
(c) Both aldehydes and ketones undergo a number of nucleophilic addition reactions.– Both aldehydes and ketones undergo nucleophilic addition. It is because both compounds have a carbonyl group,>C=O. The oxygen atom in the carbonyl group is electronegative. The bonded pairs of electrons are more towards the oxygen than the carbon atom
(ii) An organic compound with the molecular formula C7H6O gets oxidised by Tollens’ reagent. It does not respond to Fehling test but can undergo the Cannizzaro reaction. Identify the compound. Show how you used the above information to identify the compound
Ans: An organic compound is not reduced by fehling solution but undergoes cannizzaro reaction means it is benzaldehyde
Question 20
(i) When one mole of an isomer of the complex [Cr(H2O)6]Cl3 is treated with AgNO3, it produces 1 mole of a white precipitate of AgCl. Write the formula of this isomer of the complex and show how the metal-ligand bonding differs in the isomers.
Ans: The isomer of [Cr(H2O)6]Cl3 that yields one mole of AgCl when reacted with AgNO3 is [Cr(H2O)5Cl]Cl2. This isomer differs from the original complex in that one chloride ion is a ligand directly bonded to the chromium, while two chloride ions exist as counter ions.
(ii) A coordination compound shows d2sp3 hybridisation. Identify the nature of ligand as weak or strong. What will be the geometry of the compound?
Ans: A complex involving d2sp3− hybridization have octahedral geometry. Strong field ligands force the unpaired electrons of central metal ion to pair up causing d2sp3 hybridisation whereas weak field ligands do not affect electronic configuration of the metal ion undergoes in sp3d2 hybridisation.
Question 21
(i) (a) Calculate the value of Eo cell and ∆Go that can be obtained from the following cell under the standard conditions at 25°C
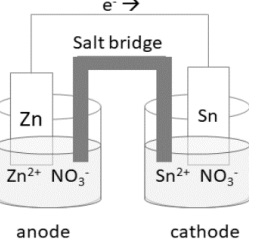
Given Eo Zn2+/Zn = -0.76V; Eo Sn2+/Sn = -0.14V, 1 Faraday = 96500 C mol
Ans 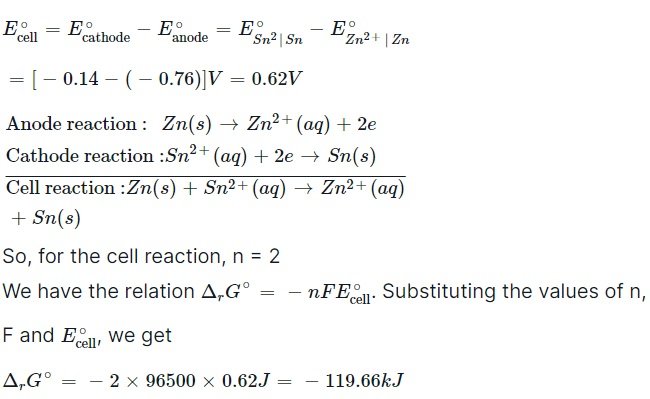
(b) How much electricity in Faraday is required for the complete reduction of MnO4 −ions present in 500 ml of 0ˑ5 M solution to Mn2+?
Ans: 1 faraday = 96500 coulombs. If we reduce MnO4- to Mn+2 we will require a total of 5 electrons for each molecule of MnO4- .
So for one molecule number of electrons needed = 5
For one mole of molecules number of electrons needed = 5 × 6.022 ×10^23 ~ 3.011×10^24 electrons
Now charge on one electron = 1.6×10^-19
Total charge on these number of electrons= Y (calculate)
Charge in faraday = Y/96500
(ii) (a) The molar conductivity vs √C curve for Na2SO4, H2SO4, and NH4OH are shown below in random order. Identify the curve that corresponds to Na2SO4, H2SO4, and NH4OH. Justify your answer.
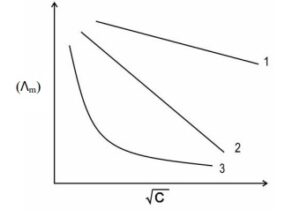
Ans : In any graph of molar conductivity vs √C,
- a weak electrolyte gives a curved decreasing graph
- strong electrolytes give straight-line decreasing graphs, in which the graph of stronger electrolyte will always be above the graph of the comparatively weaker electrolyte.
Graph 3 is curved and corresponds to Na2SO4, which is a weak electrolyte. Graph 2 & 1 respectively represent NH4OH and H2SO4l. (∵H2SO4 is a stronger electrolyte than NH4OH ).
(b) The molar conductivity (ꓥm) of a dilute solution of methanoic acid is 34ˑ1 S cm2 /mol. Calculate its degree of dissociation. (Given λ0 (H+ ) = 349ˑ6 S cm2 /mol and λ 0 (HCOO- ) = 54ˑ6 S cm2 /mol)
ꓥ0 (H+) = 349ˑ6 S cm2 /mol, λ 0 (HCOO- ) = 54ˑ6 S cm2 /mol)
λ 0 (HCOO ) = ꓥ0 (H+) + λ 0 (HCOO- )
,, = 349ˑ6 + 54ˑ6 = 404.2
degree of dissociation. = 34.1/404.2 = 0.084 = 8.4% ans
— : end of Chemistry Specimen 2024: Sec-D ISC Sample Model Paper Solved : —
–: Visit also :–
Return to : ISC Specimen Paper 2024 Solved
Thanks