Chemistry Specimen Paper 2023 Sec-A Solved for ICSE Class-10. Step by step solutions as council prescribe guideline of model sample question paper. During solutions of Chemistry Specimen Paper we explain with figure , graph, table, reaction, structure whenever necessary so that student can achieve their goal in next upcoming exam of council. Visit official website CISCE for detail information about ICSE Board Class-10.
Solutions of ICSE Class 10 Chemistry Specimen Paper 2023 Sec-A
| Board | ICSE |
| Class | 10th (x) |
| Subject | Chemistry |
| Topic | Specimen Paper Solved |
| Syllabus | Revised Syllabus |
| Session | 2022-23 |
| Section-A | With Que-1 MCQs and Q-2 one word |
| Max mark | 80 |
Warning :- before viewing solution view Question Paper. Also visit that How Board Change Same Question in Different Pattern and ask again in exam.
ICSE 2023 EXAMINATION SPECIMEN QUESTION PAPER
Chemistry
(SCIENCE PAPER – 2)
Maximum Marks: 80
- Time allowed: Two hours
- Answers to this Paper must be written on the paper provided separately.
- You will not be allowed to write during first 15 minutes.
- This time is to be spent in reading the question paper.
- The time given at the head of this Paper is the time allowed for writing the answers.
- Section A is compulsory. Attempt any four questions from Section B.
- The intended marks for questions or parts of questions are given in brackets [ ].
ICSE Chemistry Specimen Paper 2023 Sec-A Solved for Class-10
(Attempt all questions from this Section.)
Question 1: Choose one correct answer to the questions from the given options:
(i) A weak electrolyte is:
(a) Alcohol
(b} Potassium hydroxide
(c) Ammonium hydroxide
(d) Glucose
Answer : (c) Ammonium hydroxide
(ii) Electron affinity is maximum in:
(a) Alkaline earth metals
(b) Halogens
(c) Inert gases
(d) Alkali metals
Answer : (b) Halogens
(iii) The main components of bronze are:
(a) Copper and tin
(b) Copper and iron
(c) Copper and lead
(d) Copper and zinc
Answer : (a) Copper and tin
(iv) A polar covalent compound is:
(a) Methane
(b) Ammonia
(c) Nitrogen
(d) Chlorine
Answer : (b) Ammonia
(v) An acid which has two replaceable hydrogen ions:
(a) Acetic acid
(b) Hydrochloric acid
(c) Phosphoric acid
(d) Carbonic acid
Answer : (d) Carbonic acid
(vi) The hydroxide which is soluble in excess of NaOH is:
(a) Ferric hydroxide
(b) Lead hydroxide
(c) Copper hydroxide
(d) Calcium hydroxide
Answer : (b) Lead hydroxide
(vii) If the RMM of carbon dioxide is 44, then its vapour density is:
(a) 22
(b) 32
(c) 44
(d) 88
Answer : (a) 22
(viii) Drying agent used to dry Hydrogen chloride gas:
(a) Concentrated Sulphuric acid
(b) Calcium oxide
(c) Sulphurous acid
(d) Calcium hydroxide
Answer : (a) Concentrated Sulphuric acid
(ix) The catalyst used in the Haber’s Process is:
(a) Molybdenum
(b) Platinum
(c) Nickel
(d) Finely divided Iron
Answer : (d) Finely divided Iron
(x) An aqueous compound which turns colourless phenolphthalein to pink:
(a) Ammonium hydroxide
(b) Nitric acid
(c) Anhydrous calcium chloride
(d) Sulphuric acid
Answer : (a) Ammonium hydroxide
(xi) The gas formed when carbon reacts with concentrated sulphuric acid:
(a) Hydrogen
(b) Sulphur trioxide
(c) Sulphur dioxide
(d) Oxygen
Answer : (c) Sulphur dioxide
(xii) | The organic compound prepared when Ethanol undergoes dehydration:
(a) Methane
(b) Ethane
(c) Acetylene
(d) Ethene
Answer : (d) Ethene
(xiii) The IUPAC name of methyl acetylene is:
(a) Propyne
(b) Ethene
(c) Propane
(d) Ethyne
Answer : (a) Propyne
(xiv) The product formed at the cathode in electroplating of an article with Nickel is:
(a) Hydrogen gas
(b) Nickel ions
(c) Nickel atoms
(d) Oxygen gas
Answer : (c) Nickel atoms
(xv) An alkali metal found in period 3 and group 1 is:
(a) Magnesium
(b) Lithium
(c) Sodium
(d) Potassium
Answer : (c) Sodium
Question 2:
(i) The diagram shows an experiment set up for the laboratory preparation of a pungent smelling gas. The gas is alkaline in nature.
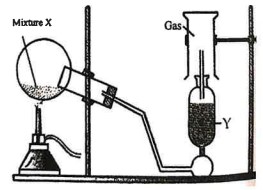
(a) Name the gas collected in the gas jar.
(b) Write a balanced chemical equation for the above preparation.
(c) How is the gas being collected?
(d) What is the purpose of using Y?
(e) How will you find that the jar is full of gas?
Answer :
(a) Ammonia
(b) 2NH4Cl + Ca(OH)2 –> CaCl2 + 2H2O + 2NH3
(c) Downward Displacement of air
(d) To get dry ammonia with lumps of quick ammonia
(e) Moist Red litmus —> Turn to Blue
(ii) Match the following Column A with Column B.
| Column I | Column II |
| (a) Acid Salt | 1. Black in colour |
| (b) Copper Oxide | 2. Reddish brown |
| (c) Zine hydroxide | 3. Hydrogen chloride |
| (d) Copper Metal | 4. Sodium Hydrogen Carbonate |
| (e) Polar compound | 5. Soluble in excess sodium hydroxide |
Answer :
(a) 4. Sodium Hydrogen Carbonate
(b) 1. Black in colour
(c) 5. Soluble in excess sodium hydroxide
(d) 2. Reddish brown
(e) 3. Hydrogen chloride
(iii) Complete the following by choosing the correct answers from the bracket:
(a) Ammonia in the liquefied form is ……… [neutral / basic]
(b) Organic compounds are generally insoluble in ……… [Water / Organic solvents]
(c) An inert electrode used in electrolysis of acidified water is ……… [iron / platinum]
(d) Hydrocarbons having double bond is ………. [alkenes / alkynes]
(e) Analkaline gas gives dense white fumes of ……… [NH4OH / NH4Cl] with hydrogen chloride gas.
Answer :
(a) neutral
(b) Water
(c) platinum
(d) alkenes
(e) NH4Cl
(iv) Identify the following:
(a) The property by which carbon bonds with itself to form a long chain.
(b) A substance that conducts electricity in molten or aqueous state.
(c) The energy required to remove an electron from the valence shell of a neutral isolated gaseous atom.
(d) The name of the process by which the Bauxite ore is concentrated.
(e) The bond formed by a shared pair of electrons with both electrons coming from the same atom.
Answer :
(a) Catenation
(b) Electrolyte
(c) Ionisation Energy
(d) Bayer’s Process
(e) Co-Ordinate Bond
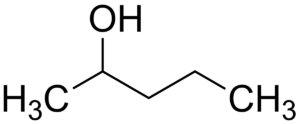
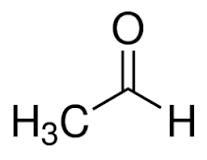
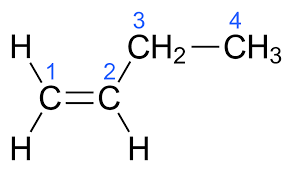
(v) (a) Draw the structural formula for the following:
1. 2-pentanol
2. Ethanal
3. 1-butene
(b) Name the following organic compounds in IUPAC system:
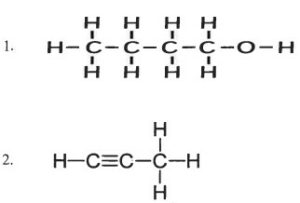
Answer :
(a)
1. 
2. 
3. 
(b)
- Butanol
- Propyne
Return to : ICSE Specimen Paper 2023 Solved
–: visit also :–
- ICSE Class-10 Textbook Solutions Syllabus Solved Paper Notes
- School will be closed on These Dates in August, Check Total Holidays
- High Salary Top Courses After 12th Science for Boys / Girls
- Which School Board is Better for IIT JEE and NEET Preparation?
- Which Entrance Exam Is Easier To Crack – NEET, IIT, JEE?.
- CISCE 2023 Exam: Clue of Board Paper Standard
- ICSE Board Paper Class-10 Solved Previous Year Question
Thanks