Chemistry Specimen Paper Sec-B 2023 Solved for ISC Class-12. Step by step solutions as council prescribe guideline of model sample question paper. During solutions of Chemistry specimen paper we explain with figure , graph, table whenever necessary.
Student can achieve their goal in next upcoming exam of council. Visit official website CISCE for detail information about ISC Board Class-12.
ISC Class-12 Chemistry Specimen Paper 2023 Solved sec-B
| Board | ISC |
| Class | 12th (xii) |
| Subject | Chemistry (Section-B) |
| Topic | ISC Specimen Paper Solved |
| Syllabus | on Revised syllabus |
| Session | 2022-23 |
ISC Class-12 Chemistry Specimen Paper 2023 Solved sec-B
Warning :- before viewing solution view Question Paper
ISC SPECIMEN QUESTION PAPER 2023
Chemistry Paper 1 (Theory)
Time Allowed: Three hours
(Candidates are allowed additional 15 minutes for only reading the paper. They must NOT start writing during this time).
- This paper is divided into four sections – A, B, C and D.
- Answer all questions.
- Section A consists of one question having sub-parts of one mark each.
- Section B consists of seven questions of two marks each.
- Section C consists of nine questions of three marks each, and
- Section D consists of three questions of five marks each.
- Internal choices have been provided in two questions each in Section B, Section C and Section D.
The intended marks for questions are given in brackets [ ]. - All working, including rough work, should be done on the same sheet as and adjacent to the rest of the answer.
- Balanced equations must be given wherever possible and diagrams where they are helpful.
- When solving numerical problems, all essential working must be shown. In working out problems, use the following data:
- Avogadro’s number = 6.023 x 10²³
SECTION B – 20 MARKS
ISC Class-12 Chemistry Specimen Paper 2023 Solved sec-B
Question 2: The osmotic pressure of 20g hemoglobin in 500ml of solution is 0·016atm at 25ºC. Calculate the molecular mass of hemoglobin.
Answer:
π =CRT
0.016 = (20/M × 0.5) × 0.0821 ×298
M = 61164.5 g
Question 3: Give reason for the following:
(i) Transition metals form large number of complex compounds.
(ii) Transition elements show variable oxidation states.
Answer:
(i) The transition metals form a large number of complex compounds. This is because: (a) Smaller size of metal ions. (b) High ionic charges. (c) Availability of empty d-orbitals for bond formation.
(ii) They show variable oxidation state because transition metals have (n-1)d orbitals empty that are closer to the outermost ns orbital in energy levels. These orbitals are never fully filled. So, they can always accommodate more electrons in (n-1)d orbitals.
Question 4: Identify compounds [A] and [B] in the following reactions.
(i) ………….
(ii) ……….
Answer:
Question 5: State reasons for the following:
(i) Ethylamine is soluble in water whereas aniline is not soluble in water.
(ii) Aliphatic amines are stronger bases than aromatic amines.
Answer:
(i) Ethylamine is soluble in water owing to its potential to form intermolecular Hydrogen bonds with water. On the other hand, aniline does not undergo hydrogen bonding because of the presence of the benzene which is highly hydrophobic. Therefore aniline is insoluble in water.
(ii) The electrons on the N-atom in aromatic amines cannot be donated easily. So, aniline is a weaker base than alkyl amines, in which +1 effect increases the electron density on the nitrogen atom. This explains why aliphatic amines are stronger bases than aromatic amines.
Question 6: Calculate the standard free energy change (∆Gº) for the following chemical reaction:
(i) ……….
(ii) ………..
Answer:
Question 7: Complete and balance the following chemical equations:
(i) ……….
(ii) ………..
Answer:
Question 8:
(i) How will the following be obtained? (Give chemical equation)
(a) Picric acid from phenol
(b) Ethanol from formaldehyde
OR
(ii) Write the chemical equations for the dehydration of ethanol with conc. H2SO4 at 140ºC and 170ºC.
Answer:
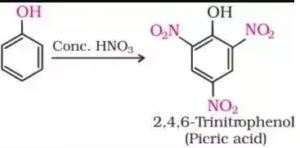
(i) (a) Picric acid is obtained by Nitration of phenol by concentrated HNO3 in presence of Concentrated H2SO4.
(b) HCHO + A —> CH3CH2OH.
Here, A is CH3MgBr.
HCHO + CH3MgBr → CH3CH2OMgBr + H2O → CH3CH2OH + Mg(OH)Br
(ii) …2C2H5OH + C2H5 − → O−C2H5 + H2O………..
Question 9: A solution of urea in water has boiling point 100·128ºC. Calculate the freezing point of the same solution. Molal constants for water are Kb = 0·512 K kg mol-1 and Kf = 1·86 K kg mol-1 respectively.
Answer:
Question 10: Give one chemical test for each to distinguish between the following pair of compounds.
(i) Formaldehyde and acetic acid
(ii) Acetaldehyde and acetone
Answer:
(i) When formaldehyde and acetaldehyde are treated with iodine in NaOH. Acetaldehyde gives yellow color precipitation. But on the other hand, formaldehyde does not react with it
(ii) Acetaldehyde and acetone can be distinguished by iodoform test
Question 11: Why are Zn, Cd and Hg not regarded as transition elements?
Answer: Transition metals are defined as the elements having incompletely filled d orbitals. Since Zn, Cd and Hg have completely filled d orbital, they are not regarded as transition elements.
–: End of Chemistry Specimen Paper Sec-B 2023 Solved for ISC Class-12 :–
–: Visit also :–
Return to : ICSE Specimen Paper 2023 Solved
Thanks


