Chemistry Specimen Paper Sec-C 2023 Solved for ISC Class-12. Step by step solutions as council prescribe guideline of model sample question paper. During solutions of Chemistry specimen paper we explain with figure , graph, table whenever necessary.
Student can achieve their goal in next upcoming exam of council. Visit official website CISCE for detail information about ISC Board Class-12.
ISC Class-12 Chemistry Specimen Paper 2023 Solved sec-C
| Board | ISC |
| Class | 12th (xii) |
| Subject | Chemistry (Section-C) |
| Topic | ISC Specimen Paper Solved |
| Syllabus | on Revised syllabus |
| Session | 2022-23 |
ISC Class-12 Chemistry Specimen Paper 2023 Solved sec-C
Warning :- before viewing solution view Question Paper
ISC SPECIMEN QUESTION PAPER 2023
Chemistry Paper 1 (Theory)
Time Allowed: Three hours
(Candidates are allowed additional 15 minutes for only reading the paper. They must NOT start writing during this time).
- This paper is divided into four sections – A, B, C and D.
- Answer all questions.
- Section A consists of one question having sub-parts of one mark each.
- Section B consists of seven questions of two marks each.
- Section C consists of nine questions of three marks each, and
- Section D consists of three questions of five marks each.
- Internal choices have been provided in two questions each in Section B, Section C and Section D.
The intended marks for questions are given in brackets [ ]. - All working, including rough work, should be done on the same sheet as and adjacent to the rest of the answer.
- Balanced equations must be given wherever possible and diagrams where they are helpful.
- When solving numerical problems, all essential working must be shown. In working out problems, use the following data:
- Avogadro’s number = 6.023 x 10²³
SECTION C – 21 MARKS
ISC Class-12 Chemistry Specimen Paper 2023 Solved sec-C
Question 12: The rate constant for a first order reaction becomes six times when the temperature is increased from 350 K to 410 K. Calculate activation energy (Ea) for the reaction.
Answer:
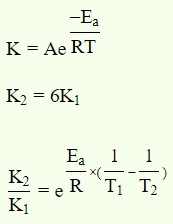
Question 13: An organic compound ‘A’ on treatment with aq .KCN produces compound ‘B’ Compound ‘B’ on reduction with Na/C2H5OH gives compound ‘C’ with molecular formula C2H7N Compound ‘C’ reacts with NaNO2 and HCl to form compound ‘D’ Compound ‘D’ on treatment with acetic acid in presence of conc. H2SO4 produces a sweet smelling compound ‘E’.
(i) Identify the compounds ‘A’ to ‘E’.
(ii) Name the reaction for the formation of compound ‘E’ from compound ‘D’.
Answer: update soon
Question 14:
(i) Name the four bases present in DNA. Which one of these is not present in RNA?
(ii) Deficiency of which vitamin causes the following diseases.
(a) Scurvy
(b) Night blindness
Answer:
(i) Adenine (A), guanine (G), cytosine (C), and thymine (T) are the four bases found in DNA.
thymine (T) is not present in RNA.
(ii) (a) Scurvy is caused by not having enough vitamin C in your diet for at least 3 months. Vitamin C is mainly found in fruit and vegetables. Even people who do not eat very healthily all the time are not usually considered at risk of scurvy.
(b) Night blindness is one of the first signs of vitamin A deficiency.
Question 15: An aqueous solution containing 12·48g of barium chloride in 1000g of water boils at 373·0832K. Calculate the degree of dissociation (α) of barium chloride.
Kb for H2O = 0·52K kg mol -1 , molecular mass of BaCl2 = 208·34 g mol -1.
Answer:
Current boiling pt =373.0832 k
Actual boiling pt =378 k
ΔT=373.0832−373
=0.0832
Now,
n of BaCl2 = mass/molar mass
=208.3412.48
≈0.0599, i.e 0.06
∴molality= n of moles/ man of sales (inkg)
=0.06
Now,
ΔTb=kb×m×i (i= vant- half factor)
⇒0.0832=0.52×0.06×i
⇒i≈ 0.0832/0.52×0.06
≈2.67
⇒1+(n−1)×α=2.67 (α degree of diss)
⇒1+2α=2.67
⇒2α=1.67
α≈0.835
Question 16: Write the chemical equation for the following named organic reactions.
(i) Haloform reaction
(ii) Reimer – Tiemann reaction
(iii) Kolbe – Schmidt reaction or Kolbe reaction
Answer:
(i) the haloform reaction is a chemical reaction in which a haloform (CHX 3, where X is a halogen) is produced by the exhaustive halogenation of an acetyl group ( R−C(=O)CH 3, where R can be either a hydrogen atom, an alkyl or an aryl group), in the presence of a base.
(ii) When phenol is treated with chloroform in the presence of sodium hydroxide, a group is introduced at the ortho position of the benzene ring. This reaction is known as the Reimer-Tiemann reaction. The intermediate is hydrolyzed in presence of alkalis to produce salicylaldehyde.
(iii) When phenol is treated with sodium hydroxide, sodium phenoxide is produced, which on treating with carbon dioxide, followed by acidification, undergoest electrophilic substitution to give a hydroxybenzene acid as the main product
Question 17:
(i) Identify the compounds A, B and C in the following reactions:
(a) ………….
(b) ………
(ii) How will the following be converted? (Give chemical equations)
(a) Benzenediazonium chloride to Benzene
Ans:- When benzene diazonium chloride is treated with benzene diazonium chloride with hypophosphorous acid in presence of cuprous ions, benzene is obtained
(b) Ethylamine to ethyl alcohol
Ans: At a temperature of 0 to 5 degree celsius, ethyl amine reacts with a Nitrous acid(obtained from NaNO2solution and dilute HCl),to give ethyl diazonium chloride. This can be given a little heat in a aqueous medium to give ethanol
(c) Methylamine to methyl isocyanide
Ans: The common method for preparing methyl isocyanides is the dehydration of N-methylformamide.
Question 18: Suppose 50 bacteria are placed in a flask containing nutrients, so that they can multiply. A study at 35 C gave the following results:
Answer the following questions:
(i) This multiplication of bacteria follows:
(a) Zero order reaction
(b) First order reaction
(c) Second order reaction
(d) Third order reaction
Ans: (b) First order reaction
(ii) The rate constant for the reaction is:
(a) 0·0462 min-1
(b) 0·462 min-1
(c) 4·62 min-1
(d) 46·2 min-1
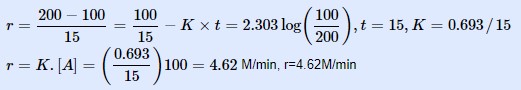
Ans: (c) 4·62 min-1
(iii) The half life period (t1/2) of the reaction is:
(a) 1500 minutes
(b) 150 minutes
(c) 15 minutes
(d) 1·5 minutes
Answer:
–: End of Chemistry Specimen Paper Sec-C 2023 Solved for ISC Class-12 :–
–: Visit also :–
Return to : ICSE Specimen Paper 2023 Solved
Thanks


