Class-10 Analytical Chemistry Goyal Brothers ICSE Solutions Ch-4. Step by Step Solutions of Exercise and Objective Type Questions of Goyal Brothers Prakashan Chapter-4 Analytical Chemistry for ICSE Class 10 . Visit official Website CISCE for detail information about ICSE Board Class-10 Chemistry .
Class-10 Analytical Chemistry Goyal Brothers ICSE Solutions Ch-4
-: Select Topics :-
Objective Type Questions (update soon)
The determination of chemical components of a substance is called analysis of that substance. The analysis can be either Quantitative or Qualitative.
Quantitative analysis involves the determination of the composition of a mixture, whereas, qualitative analysis involves the identification of an unknown substance in a mixture.
Identification of unknown substances during the qualitative analysis is done with the help of reagents. (Sodium Hydroxide and Ammonium Hydroxide in case ICSE Class-10 Syllabus) .
Exercise Class-10 Analytical Chemistry Goyal Brothers ICSE Solutions Ch-4
Question 1. You are provided with the following salt solutions in separate test tubes
(i) aqueous FeSO4
(ii) aqueous FeCl3
(iii) aqueous Mg(NO3)2
(iv) aqueous ZnSO4
(v) aqueous lead nitrate
(vi) aqueous copper sulphate.
(a) What will you observe in case of each salt solution when sodium hydroxide solution is added
(i) in small amount.
(ii) In excess? Support your answer by relevant chemical equations.
(b) What will you observe in case of each salt solution, when ammonium hydroxide solution is added
(1) in small amount,
(2) in excess? Support your answer by relevant chemical equations.
Answer:
(a) What will you observe in case of each salt solution when sodium hydroxide solution is added
(i) in small amount. (ii) in excess
Answer:
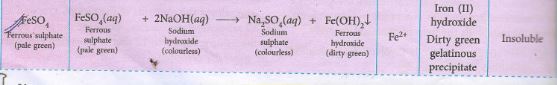

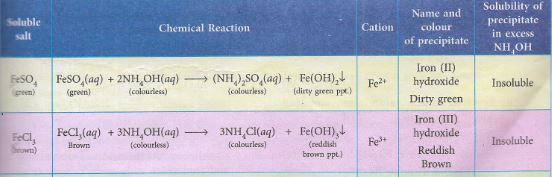
(b) What will you observe in case of each salt solution when Ammonium hydroxide solution is added
(i) in small amount. (ii) in excess
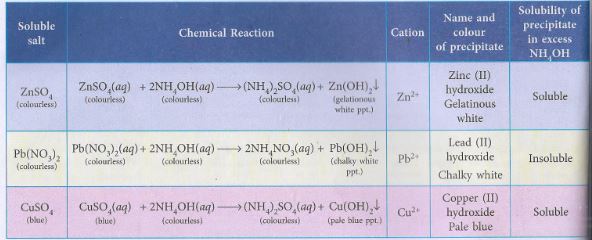
Question 2. Zinc and aluminium metals are amphoteric in nature, what do you understand by the statement? Support your answer
by writing fully balanced equations for zinc and aluminium.
Answer
Reactions of Zinc with acid and base follows:
With acid:
With base:
When zinc metal reacts with sodium hydroxide, it produces sodium zincate and hydrogen gas.
Zinc with acid and base produces a salt and releases hydrogen gas.
Reactions of Aluminium with acid and base follows:
With acid:
When aluminium reacts with hydrochloric acid, it produces aluminium chloride and hydrogen gas.
With base:
When aluminium reacts with sodium hydroxide, it produces sodium aluminate and hydrogen gas.
Aluminium with acid and base produces a salt and releases hydrogen gas.
Question 3. The oxides and hydroxides of zinc and aluminium metals are amphoteric in nature. What do you understand by the statement? Support your answer by writing fully balanced equations for the oxides and hydroxides of zinc and aluminium.
Answer
Zinc oxide (ZnO) reacts with both acids and with bases:
- In acid: ZnO + H2SO4 → ZnSO4 + H2O
- In base: ZnO + 2 NaOH + H2O → Na2[Zn(OH)4]
Aluminium oxide (Al2O3) reacts with both acids and with bases
- In acid: Al2O3 + 6 HCl→ 2 AlCl3 + 3 H2O
- In base: Al2O3 + 2 NaOH + 3 H2O → 2 Na[Al(OH)4] (hydrated
Aluminium hydroxide reacts with both acids and with bases
- As a base (neutralizing an acid): Al(OH)3 + 3 HCl → AlCl3 + 3 H2O
- As an acid (neutralizing a base): Al(OH)3 + NaOH → Na[Al(OH)4]
Zinc hydroxide reacts with both acids and with bases
Question 4. Write the colour of the following salts:
(a) Aluminium salts (b) Calcium salts (c) Cupric salts (d) Ammonium salts (e) Ferrous salts
Answer:
(a) Aluminium salts –Colourless
(b) Calcium salts—Colourless
(c) Cupric salts —Blue
(d) Ammonium salts—Colourless
(e) Ferrous salts—Light green
Question 5. What happens when sodium hydroxide solution is added first dropwise and then in excess to the following solution:
(a) FeCl2 (b) Mg(NO3)2 (c) CuSO4 (d) ZnSO4
Answer:
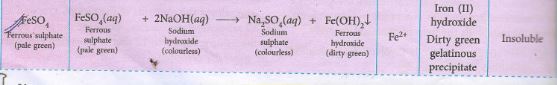

Objective Type Questions
I. Multiple Choice Questions
Choose the correct answer from the options given below :
- ………………
- ………………
- …………….
- ………………..
- …………………..
- ………………….
- ……………………
- …………………..
I. Fill in the blanks spaces with the choice given in brackets:
- …………………..
- ……………………..
- …………………
- …………………
- ………………..
- ………………..
–: end of Analytical Chemistry Solutions Goyal Brothers :–
Return of : Chemistry Class-10 Goyal Brothers Prakashan
Thanks
Share with your friends

Pls provide the answer of MCQ and other left over questions fast
ok
we will try
Pls provide mcq and fill ups answers too
ok very soon
Pls provide MCQ sir
we will try mcq also
Please send objectives
ok