Class-6 Dalal Air Atmosphere ICSE New Simplified Chemistry Solutions. Dr Viraf J Dalal Middle School Allied Publishers Solutions. Chapter-5. We Provide Step by Step Solutions of Exercise/Lesson -5 Elements Compound and Mixtures with Objective Type Questions, Fill in the blanks and Give reason , Match the following of Dr Viraf J Dalal Middle School Chemistry Allied Publishers. Visit official Website CISCE for detail information about ICSE Board Class-6.
Class-6 Dalal Air Atmosphere ICSE New Simplified Chemistry Solutions Chapter-5
EXERCISE-5
Question 1.
State what do you understand by the term ‘air’. Explain its importance to mankind.
Answer 1:
A mixture which occupies space has mass, and whose composition is differ at places to pace and time to time is called Air
Importance of Air to mankind.
- Air is essential for respiration living beings
- For burning
- for tyre pressure
- for sound propagation
Question -2.
Give a brief account of the discovery of air and the scientists involved.
Answer -2:
- John Mayow – Air has two components i.e. active and inactive
- Lavoiser – Active component of air is oxygen and an inactive component of air is nitrogen.
- Other scientists discovered noble gases and carbon dioxide., CO2, water vapours.
Question- 3.
What is meant by the term ‘atmosphere’. State the role played by the atmosphere for the survival of mankind.
Answer -3:
A layer/Blanket above the earth which spreads like a blanket of air is called atmosphere.
role played by the atmosphere for the survival of mankind.
- The atmosphere protects us from various harmful gases and air would not exist without the atmosphere.
- It also keeps the earth warm at nights and provides gases like carbon dioxide and nitrogen to plants .
Question -4.
Explain in brief the different layers of the atmosphere and the basic functions of the main layers.
Answer -4:
layers of the atmosphere
- Troposphere -Troposphere is the layer just above the earth’s surface whose distance is between 0 – 10km. It contains most of the air and oxygen which is essential for all living organisms. This air is used for respiration and other functions for the survival of human beings. It also forms the weather conditions of the earth.
- Stratosphere – Stratosphere is The layer above the troposphere at a distance between 10 – 50km above the earth’s surface. It consists of the ozone layer which protects the earth from ultraviolet rays of the sun.
- Mesosphere – Mesosphere The layer above stratosphere at a distance between 50 – 80km above the earth’s surface. This layer is very cold and burns most of the meteors preventing them from reaching the earth’s surface.
- Thermosphere – layer above the mesosphere at a distance between 80 – 320km above the earth’s surface is Thermosphere .
- Exosphere:– Top most layer is called Exosphere:

Question -5.
Describe a simple experiment with the help of a diagram to show that :
(a) Air occupies space
(b) Air has mass
(c) Air exerts pressure
(d) Air is highly compressible
Answer -5:
(a) Experiment – To show air occupies space
Procedure – A glass bowl is half filled with water. An empty glass tumbler is inverted inside the bowl.
When the tumbler is tilted, bubbles are seen in water.

Observation – The air was occupying space inside the tumbler, and on tilting air is displaced and bubbles come out because of that.
Conclusion – Air occupies space.
(b) Experiment – To show air has mass
Procedure – An inflated balloon is placed on one side of a simple scale, and the scale tilts towards one side.

Observation – The balloon has mass which makes the scale tilt.
Conclusion – Air has mass.
(c) Experiment – To show air exerts pressure.
Procedure – An open can is filled with water and heated. On the formation of steam, the cap is placed tightly such that steam is inside the can.

Observation – The steam condenses and the pressure inside reduces as the steam reduces. The air outside the can exerts pressure and makes the can crumple.
Conclusion – Air exerts pressure.
(d) Experiment – To show air is highly compressible.
Procedure – A leak proof syringe which is empty and filled with air is pushed towards the end.

Observation – The piston of the syringe is moved inwards, which implies the air inside the syringe is compressible.
Conclusion – Air is highly compressible.
Question- 6.
Name the components of air with their approximate percentage by volume in air. Does the percentage by volume of each component remain the same, in the atmosphere of different parts of the world. Explain with reasons.
Answer -6:
Main components of air are :
- Nitrogen
- Oxygen
Composition of air
- Nitrogen – 78%
- Oxygen 21%
- Carbon dioxide 0.03 – 0.04%
- Inert gases 0.9%
- water vapour- variable
Composition of dust, water vapour and impurities is different in different parts of the world, depending upon the factors affecting the air like industrial activities, natural resources, etc.
Question -7.
With the help of a labeled diagram, describe a simple experiment to show the presence of oxygen and nitrogen in air using a piece of white phosphorus.
Answer- 7:
Experiment – to show oxygen and nitrogen component in air using a bell jar.
Apparatus – a trough, a bell jar, a cork, a crucible and a piece of white phosphorus.


Procedure – A trough is filled with and a bell jar is labelled into 5 equal parts.
A crucible which has white phosphorus is placed on a cork is floating in the water.
The level of water inside the jar and outside the jar are adjusted to one level
The phosphorus is ignited by means of an ignited wire.
Observation – The phosphorus burns in air with oxygen and forms dense white fumes of phosphorus pentoxide.
The level of water inside the bell jar rises by 1/5th.
Conclusion – Oxygen is the active component in air which burns phosphorus and nitrogen is the inactive component of air which is not used in burning.
Question -8.
You are given a test tube with two outlets and a bottle of lime water. Using the same, how would you demonstrate experimentally the presence of carbon dioxide in air.
Answer -8:
Procedure – Lime water is added to the test tube with two outlets attached to it.
Air is sucked slowly from one outlet.
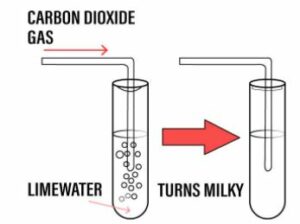
Observation – The lime water in the test tube turns milky
Conclusion – Air which is sucked contains carbon dioxide which turns lime water milky.
Question -9.
Give a reason why water droplets appear on the outer surface of a tumbler containing ice.
Answer- 9:
Water droplets appear on the outer surface of the beaker. Water vapour condenses on the cooler surface of the beaker, which proves air contains water vapour.
Question -10.
Explain the importance of nitrogen of the air for plant growth.
Answer -10:
Nitrogen is converted into soluble nitrogenous compounds in soil because of air and moisture. These compounds are absorbed by plants and used as proteins.
Question -11.
Give a reason why nitrogen is filled in food packets and not oxygen.
Answer -11:
Nitrogen is an inert and unreactive gas which is filled in food packets to remove oxygen and moisture from them.
It keeps the food preserved and fresh by reducing the bacterial growth in the food packages.
Question -12.
State what would happen, if the air above the earth contained mainly oxygen and no nitrogen.
Answer -12:
Nitrogen is utilised by plants for their growth and development. It is converted to soluble nitrogenous compounds in the soil in the presence of air and moisture. They are absorbed by plants and utilised as plant proteins. With no nitrogen, plants will not be able to survive on earth.
Question -13.
State the utility of oxygen for respiration in
- Living organisms
- Plants
Answer 13:
1) Respiration of living organisms involves inhaling oxygen to carbon & hydrogen present in food material which forms carbon dioxide , water vapor and energy in the form of heat.
Glucose + Oxygen → Carbon dioxide + water vapour + Energy
2.) Plant respiration is the process of plants using up the sugars made through photosynthesis and turning them into energy for growth, reproduction, and other life processes. Plant respiration involves taking in oxygen and releasing it as carbon dioxide into the atmosphere (air).
In photosynthesis, solar energy is harvested as chemical energy in a process that converts water and carbon dioxide to glucose. Oxygen is released as a byproduct. In cellular respiration, oxygen is used to break down glucose, releasing chemical energy and heat in the process.
Question- 14.
Compare respiration and combustion-both involving oxygen of the air.
Answer- 14:
Respiration is a process whereby living organisms
- Use oxygen from the air to oxidize food substances mainly glucose, in their body cells.
- Release energy in the form of heat.
- Carbon dioxide and water vapour are also produced and released in the exhaled air.

Combustion or burning, involves oxidation i.e. combination of substances like fuels with oxygen or air generally resulting in production of heat and light.

Carbon dioxide is released into the air as a result of all burning.
Question -15.
Explain the importance of carbon dioxide for
(a) photosynthesis
(b) warming the earth’s environment.
State what would happen if excess carbon dioxide as a pollutant is released into the atmosphere.
Answer- 15:
(a) In photosynthesis, green plants manufacture their own food by absorbing carbon dioxide in the air and make food in the presence of chlorophyll and sunlight.

(b) In the greenhouse effect, earth gets heated up because of infrared, visible ray & ultra violet rays. Carbon dioxide in the air allows UV rays to pass through but prevents the infrared rays from being radiated out of earth’s surface. It results in warming the earth’s environment.
If excess carbon dioxide is released into the atmosphere, it will result in rise in temperature leading to global warming.
Question -16.
State in brief how water vapour in the atmosphere determines the climatic conditions.
Answer- 16:
Water vapour in the atmosphere determines climatic conditions by:
- Minimizing the rate at which evaporation takes place.
- Condensation of clouds into rain, and thereby dropping the temperature.
Question- 17.
Give a reason why two different rare (inert) gases find application in advertisement signs and fluorescent bulbs.
Answer -17:
- Neon –Neon is used in advertisement signs because when current is passed through this gas at low pressure, a brilliant red glow can be seen. This colour can be changed by adding mercury and argon.
- Xenon –Xenon is used in fluorescent bulbs and lasers because it emits white light in discharge tubes.
Question- 18.
A mixture has a variable composition. Give three other reasons why air is considered a mixture and not a compound.
Answer -18:
Air is a mixture and not a compound because:
- Air does not have a formula like a mixture, while compounds have a fixed formula.
- When air is formed by gases, there is no energy change.
- Properties of air are variable and subjective to time and place.
- Components of air can be physically separated.
Question -19.
State a reason why there is a balance in the amount of carbon dioxide in the air, even though carbon dioxide is released into the atmosphere by various processes.
Answer- 19:
In photosynthesis, green plants manufacture their own food by absorbing carbon dioxide in the air and make food in the presence of chlorophyll and sunlight.
![]()
![]()
Oxygen is released into the atmosphere, which maintains the composition of air and does not increase the amount of carbon dioxide in air.
Question -20.
Name three different appliances where air is utilized.
Answer- 20:
- Vehicles – Run on tyres which are inflated with air.
- Aeroplane – Aeroplane fly in air creating heavy pressure deference on wings
- Brake mechanisms – Of trains and other machines, work on compressed air.
- Pumps and siphons – Work on air pressure.
Objective Type Questions
Class-6 Dalal Air & Atmosphere ICSE New Simplified Chemistry Solutions Chapter-5
Question-.1.
Match the statements in List I with the correct answers in List II.
| List I | List II |
|---|---|
| An impurity in air | A: Water vapour |
| The component of air which supports combustion | B: Respiration |
| The inert component in air, slightly soluble in water | C: Rare gases |
| The component of air which minimizes the rate of evaporation | D: Sulphur dioxide |
| The process which involves oxidation of food substance in living organisms | E: Helium |
| The component of air which is heavier than air and fairly soluble in water | F: Carbon dioxide |
| The process which involves oxidation of substances, liberating heat and light energy | G: Combustion |
| The component of air which is heavier than air and fairly soluble in water | H: Nitrogen |
| The process by which atmospheric carbon dioxide is absorbed by plants resulting in formation of carbohydrates | I: Photosynthesis |
| The component of air, which is lighter than air and less than 1% in air | J: Oxygen |
Answer-1:
- D:
- J:
- H:
- A:
- B:
- E:
- G:
- F:
- I:
- C:
Q.uestion-2.
The diagrams below represents an experiment to show the presence of a – component of air.

- State why air is sucked from – outlet ‘B’ and not from outlet ‘A’.
- State why air is sucked ‘slowly’ from the outlet ‘B’
- State the observation after completion of the experiment.
- State the reason for the above observation seen.
- Name another component which if present in air as a pollutant – would have given a similar observation.
Answer-2
- Sucking from outlet ‘A’ will suck lime water out.
- Sucking air quickly will suck lime water out.
- Lime water turns milky.
- Formation of CO2 inside the test tube.
- Sulphur dioxide
Question-.3.
Complete the statements given below by filling in the blanks with the correct word/s from the word/s in bracket.
- The ___ (atmosphere/troposphere/stratosphere) is a layer which extends up to about 10-15 km above the earth.
- _ (helium/hydrogen sulphide) is an example of a pollutant present in air.
- The component of air used in photosynthesis is _______ and the products of photosynthesis is ____ ( oxygen/carbon dioxide/nitrogen)
- The product formed which is common to both combustion and respiration is ______ (oxygen/carbon dioxide/ nitrogen dioxide)
- The component of air which is variable in air above sea level and in air in general ______ (oxygen/nitrogen/water vapour)
Answer-3
- Stratosphere
- Hydrogen sulphide
- Carbon dioxide
- Nitrogen dioxide
- Water vapour
Question-.4.
Name the following :
- The product formed when phosphorus burns in oxygen.
- The layer of atmosphere which contains the ozone layer.
- The active component of air which supports combustion and is used up in burning.
- The main, rare(inert) gas present in the air.
- The component of air which is present more in industrial areas.
- The type of bacteria which directly absorb nitrogen from the air and converted soluble nitrates in the soil.
- The product of respiration released in exhaled air, other than carbon dioxide.
- An inert gas which has a low boiling point and is used for producing very low temperatures.
- A process which removes carbon dioxide from the air.
- The component of air which does not support combustion.
Answer-4
- Phosphorus pentoxide
- Stratosphere
- Oxygen
- Argon
- Carbon monoxide, sulphur dioxide, oxides of nitrogen, hydrogen sulphide, dust particles.
- Symbiotic bacteria
- Water vapour and energy
- Helium
- Photosynthesis
- Nitrogen
Question-.5.
State whether the following statements are ‘true’ or ‘false’. If false write the correct statement.
- The mesosphere in the atmosphere contains most of the air, fit for respiration.
- The percentage of carbon dioxide in the air is between 0.2 to 0.4%
- Carbon dioxide, water vapour and energy in the form of heat are evolved during respiration.
- Photosynthesis is a process by which green plants prepare their own food in the presence of sunlight and chlorophyll
- Air is a mixture and not a compound since the components of air cannot be separated by physical methods.
Answer-5
- False, The troposphere in the atmosphere, contains most of the air, fit for respiration.
- True
- True
- True
- False, air is a mixture and not a compound because its components can be separated by physical methods.
Question-.6.
Give word equations for the following conversions.
- Nitrogen of the air to nitric oxide.
- Nitric oxide to nitrogen dioxide.
- Nitrogen dioxide to nitric acid.
- Nitric acid to soluble nitrates in the soil.
- Carbon dioxide in the air to carbohydrates in plants.
Answer-6
1.)
![]()
2.)
Nitric oxide + Oxygen → Nitrogen dioxide
Nitric oxide reacts with oxygen to form nitrogen dioxide.
3.)
Nitrogen dioxide + Oxygen + Water → Nitric acid
Nitrogen dioxide reacts with oxygen and water to form nitric acid.
4)
Calcium carbonate + Nitric acid → Calcium nitrate + Water + Carbon dioxide
5).

.– : End of Class-6 Air & Atmosphere Dalal Simplified Solutions :–
Return to – Dalal Simplified Chemistry for ICSE Class-6 Solutions
Thanks
Share with your friends.


