Class-6 Dalal Water ICSE New Simplified Chemistry Solutions. Dr Viraf J Dalal Middle School Allied Publishers Solutions. Chapter-4. We Provide Step by Step Solutions of Exercise/Lesson -4 Elements Compound and Mixtures with Objective Type Questions, Fill in the blanks and Give reason , Match the following of Dr Viraf J Dalal Middle School Chemistry Allied Publishers. Visit official Website CISCE for detail information about ICSE Board Class-6.
Class-6 Dalal Water ICSE New Simplified Chemistry Solutions Chapter-4
EXERCISE-4
Question 1.
State the sources of water
(a) on the earth’s surface
(b) below the earth’s surface.
Answer 1:
- On the earth’s surface – rain water, river, lake, sea Snow, frost,
- Below the earth’s surface – underground well, spring
Question 2.
Give the importance of water in
(a) life processes
(b) household purpose
(c) fire fighting
(d) transportation.
Answer 2:
The importance of the Water is as follows :
(a) Life processes — Water is used by all living being for carrying various metabolic processes including photosynthesis & excretion .
(b) Household purposes —Water is used for cooking, bathing, washing and watering plants.
(c) Fire fighting — Water is used for extinguishing fires either directly or as a constituent in a fire extinguisher.
(d) Transportation — Water serves as a habitat for marine life i.e. preferred place for an organism to live.
Question -3.
Explain how water plays an important role in
(a) industry
(b) agriculture.
Answer- 3:
(a) Uses of Water in Industry :
- generates electricity
- Water generates steam in boilers,
- in chemical & other industries for cooling & cleaning operations.
(b) Uses of Water in Agriculture :
- It is used for irrigation of fields.
- It is used for production and growth of crops.
- It is used as a medium for spraying pesticides.
Question -4.
Give the occurrence of water in the three different states i.e. solid, liquid and gaseous.
Answer- 4:
The Occurrence of water in three different states are :
- Solid state — snow and frost.
- Liquid state — sea , river and lake Ponds
- Gaseous state — water vapour in air
Question -5.
Draw a labelled diagram to show the change of state of water from solid state to liquid state to gaseous state starting from ice.
Answer -5:
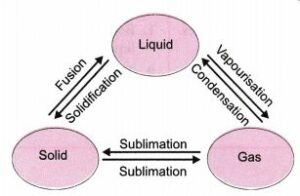
Question -6.
Explain the term water cycle. State the main points to show how water moves from the earth’s surface to the atmosphere and back to the earth’s surface as rain.
Answer 6:
The natural process of the circulation of water from the earth’s surface to the atmosphere and then back to the earth’s surface is called a water cycle.
From earth’s surface to the atmosphere:
- Evaporation – The water in water bodies evaporates because of the heat from the sun and forms clouds in the atmosphere from the water vapour.
- Respiration by living beings – Glucose + Oxygen → Carbon dioxide + Water + energy
- Burning of fuels – Fuel + oxygen → Carbon dioxide + water + energy
From atmosphere to earth’s surface:
- Rains – when the clouds formed from the water vapour in the atmosphere and become very heavy, they condense and fall down as rain.
- Steam Condense in winter – In winter vapour condense into water on surface
Question -7.
Give a reason why water is considered a universal solvent.
Answer -7:
due to a polar covalent compound. When water comes in contact with any substance it breaks the electrostatic forces holding the molecules of that substance. hence dissolve in water. So, water is called a universal solvent
Question -8.
Define the term –
(a) solute
(b) solvent
(c) solution with reference to addition of sodium chloride to water.
Answer- 8:
- Solute – The substance which is lesser amount dissolving in a solution, (Sodium Chloride)
- Solvent – The substance which is in more amount are dissolved to solute , (Water)
- Solution – The mixture formed after solute dissolves in the solvent. (Salty Syrup)
Question -9.
Draw a neat labelled diagram of addition of copper sulphate to water. Label the solute, solvent and solution in the same.
Answer -9:
Please refer figure with your text book
Question- 10.
From the following substances given below state which will form a solution in the same.
(a) sodium carbonate
(b) calcium carbonate
(c) charcoal powder
(d) sodium sulphate
(e) table salt
(f) powdered particles of lead from a lead pencil
(g) iron powder
(h) copper filings
(i) sand particles
(j) honey
Answer 10:
(a) sodium carbonate
(d) sodium sulphate
(e) table salt
(j) honey
Question 11.
Name two gases each
(a) which are soluble in water
(b) which are insoluble in water.
Answer 11:
(a) Carbon dioxide and chlorine
(b) Nitrogen and hydrogen
Question -12.
If ‘X’ g. of potassium nitrate is added to 100 g. of water at 60°C and the salt dissolves completely then —
(a) is ‘X’ g. the solubility of potassium nitrate at 60°C.
(b) is the solution formed – saturated or unsaturated
(c) if on addition ‘X’ + ‘Y’ g. of potassium nitrate to the same amount of water at the same temperature and the solute now just remains behind after stirring then –
(d) is the solution now – saturated or unsaturated
(e) is ‘X’ + ‘Y’ g. the solubility of potassium nitrate.
Answer -12:
Add ‘X’ g. of solute i.e. potassium nitrate to 100 g. of water 60°C.
When ‘X’ g of potassium nitrate is added to 100 g of water, and stirred well at 60°C. ‘X’ g of solute dissolves completely in water, so more solute will be added and again stirred thoroughly. The solute continues to dissolve, hence the solution is unsaturated.
Now, ‘X’ + ‘Y’ g of solute is added to water and the solute remains behind even after stirring. The solution is now saturated.
Solubility of potassium nitrate is ‘X’ + ‘Y’ g
Question -13.
State whether the following statements are true or false. If false write the correct statement.
- The solubility of most solid – decrease with increase in temperature.
- Distilled water is portable water
- The process to remove germs in water is also called sterilization.
Answer-13
- False, Solubility increases with temperature for most solids.
- False, Drinking water is potable water.
- True
Question- 14.
Differentiate between chemical pollution and thermal pollution.
Answer -14:
| Chemical pollution | Thermal pollution |
|---|---|
| When a large number of industrial chemicals are disposed of in water bodies and pollute water, it is chemical pollution. | Hot water from chemical plants in the iron & steel industry is discharged into streams. |
| Chemical pollutants are metallic salt solutions, mercury, lead. | This enhances the growth of harmful biological organisms. |
| Agricultural wastes like pesticides, fertilizers also enter water through underground penetration and also pollute water. | Solubility of oxygen decrease which effect the aquatic life for respiration |
Question -15.
State some important steps to avoid pollution of water.
Answer 15:
Some important steps to avoid pollution of water.
(a) Do not enter Harmful wastes such as oils & chemicals into the water.
(b) should be used Proper toilets & sewage systems to prevent human excreta, containing disease causing organisms to enter into the water.
(c) should be avoided Washing of clothes & bathing near water sources.
(d) to minimizes pollution. Planting of trees near water sources including river banks also
(e) the water should be cooled before being discharged as a waste To minimize thermal pollution .
(f) Man should be made aware through various awareness programmes & media about the harmful effects of water pollution & ways to control it.
Question -16.
State what is meant by the term ‘conservation of water’. State a few water saving methods, which may be used in the home to conserve water.
Answer 16:
Conservation of water :- the means of preventing wastage of water so that clean water can be obtained by preventing pollution of water and by protecting the sources of water.
Need for conservation —only a small percentage is potable water fit for human consumption and household purposes. Inspite of large quantities of water on the earth’s surface
How conserve water in the home.
(a) such as closing running taps and using smaller cisterns in toilets.
(b) checking all leakages in household pipes.
(c) turning off the water tap while brushing teeth and while washing hands.
(d) using less electricity, since power plants also consume substantial amount of water.
Question- 17.
Give a reason why :
- Conservation of water is essential in spite of the fact that three-fourth of earth’s surface is covered by water.
- Polluted water causes diseases.
- Drip irrigation helps in conservation of water.
Answer -17:
- The amount of potable water is very less which can be used for human consumption and other household purposes.
- Polluted water has a lot of harmful substances which are poisonous and cause diseases.
- Drip irrigation utilizes a very small quantity of water.
Objective Type Questions
Chemistry Solutions Chapter-4 Class-6 Dalal Water ICSE New Simplified
Question-1
Complete the statements by filling in the blanks with correct word/s:
- __ is the purest form of water. It may dissolve gases like _____ forming weak acids, (seawater/lake water/rainwater: chlorine/ammonia/carbon dioxide)
- Water generates ______ in hydroelectric power stations and _____ in boilers for industrial applications, (carbon dioxide/steam/heat/electricity)
- Water is added to the atmosphere by ____ and _____ (burning od fossil fuels/condensation of vapour/respiration by living organisms/photosynthesis)
- If potassium nitrate is added to water in a beaker to give a homogeneous mixture, they potassium nitrate is referred to as the ____ water as the ____ and the homogeneous mixture as the solution (solution solute/solvent/saturated solution)
- Water fit for human consumption and drinking purposes is called ____ (distilled water/potable water/rain harvested water)
Answer-1
- Rain water, carbon dioxide
- Electricity
- burning of fossil fuels, respiration by living organisms
- Solute, solvent
- Potable water
Question-.2.
State whether the following statements are true or false. If false, write the correct statement.
- Sea water contains salts of calcium and magnesium.
- Water finds application as a means of transporting goods
- On boiling water exists in the liquid state.
- Respiration uses up water from the atmosphere.
- Well water exists below the impervious rocket layers of earth.
- Sodium carbonate and sodium sulphate do not form an aqueous solution.
- If ‘X’ g. of solute is added to 100g of water at 1 ° and the solution formed is a saturated solution, then ‘X’ g. is the solubility of the solute.
- Purification of water is carried out to remove – dissolved gasses e.g. carbon dioxide and dissolved minerals like magnesium salts.
- Water from rivers and lakes is – portable water.
- Chemical pollutants include metallic salt solution of mercury and lead. Seawater
Answer-2
- True
- True
- False, On boiling water turns into its gaseous state.
- False, respiration releases water in the atmosphere.
- False, Well water exists above the impervious rocky layer of earth.
- False, Sodium carbonate and sodium sulphate from an aqueous solution.
- True
- True
- False, Water from rivers and lakes is impure water.
- True
Question-.3.
Match the statements in List 1,1-10 with their correct answer in List II, A to J.
| List I | List II |
|---|---|
| 1. Sodium carbonate and water | A: Sea water |
| 2. Chemical used in sedimentation during Purification of water | B: Preferred place for an organism |
| 3. Vapourisation | C: Liquid state |
| 4. Kerosene and water | D: Aqueous solution |
| 5. A solution which can dissolve more of solute At a given temperature | E: Dissolved gases and minerals |
| 6. Water as a habitat for marine life | F: Unsaturated solution |
| 7. A water-borne disease | G: immiscible mixture |
| 8. Existence of water between 0C to 100c | H: Liquid to gaseous state |
| 9. Taste in water is due to | I: Dysentery |
| 10. The most impure form of water | J: Alum |
Answer-3:
- D:
- J:
- H:
- G:
- F:
- B:
- I:
- C:
- E:
- A:
Question-.4.
Explain the meaning of the following terms :
- Rain water harvesting
- Drip irrigation in agriculture
- Saturated solution
- Conservation of water
- Gaseous state of water in air
Answer-4:
- Rain water harvesting:- utilizing rain water instead of allowing it to be wasted to be conducted by building tanks or pits in low lying areas and collecting roof top rain water through pipes into tanks.
- Drip irrigation in agriculture:- use pipe for supply of water in small quantities.
- A saturated solution :- A solution in which Solute cannot dissolve more at a given temperature.
- Conservation of water :- preventing wastage of water for future purpose
- Gaseous state of water in air : -Gaseous State / water vapour present in atmosphere
Qustion-.5.
Name the following :
- A chemical used during chlorination of water
- An agricultural pollutant in water
- A solid ‘natural form of water’
- The natural process by which circulation of water takes place from the earth’s surface to the atmosphere and back to earth surface
- The liquid or medium of dissolution which allows the solute to dissolve in it.
Answer-5
- Chlorine
- Insecticides / Pesticide
- Snow
- Water cycle
- Water as solvent
.– : End of Class-6 Water Dalal Simplified Solutions :–
Return to – Dalal Simplified Chemistry for ICSE Class-6 Solutions
Thanks
Share with your friends.

Very good work
ok thanks
whts the use of this website if you cant even show the diagrams atleast mention page number
fully working if any problem contact 8957797189