Matter and Its Composition Class-7 Dalal Simplified ICSE Chemistry Solutions Chapter-1 Dr Viraf J Dalal Middle School Allied Publishers Solutions. Chapter-1. We Provide Step by Step Solutions of Exercise/Lesson -1 Questions and Answers of Dr Viraf J Dalal Middle School Chemistry Allied Publishers. Visit official Website CISCE for detail information about ICSE Board Class-7.
Matter and Its Composition Class-7 Dalal Simplified ICSE Chemistry Solutions Chapter-1
| Board | ICSE |
| Class | 7th |
| Subject | Chemistry |
| Book Name | Dalal New Simplified |
| Chapter-1 | Matter and its Composition |
| Unit-1 | Matter and its Composition |
| Topic | Solution of exercise questions |
| Session | 2023-24 |
Exercise-1
Matter and Its Composition Class-7 Dalal Simplified ICSE Chemistry Solutions Chapter-1
Question 1. Explain the meaning of the term matter with special reference to the term ‘substance’.
Answer 1: A basic substance which makes up all the materials, living or nonliving are made is called matter.
Question 2. Name the three states of matter. On what basis are the three states classified.
Answer 2: Three states of matter are given below:
- Solid
- Liquid
- Gases
Basis of the classification of Three states of matter depend upon:
1. Compressibility
2. Rate of diffusion
3. Volume
Question 3. Each of the three states of matter has mass. Explain with the help simple experiments – that each state of matter has mass.
Answer 3: (a) Solid :A solid is placed on one side of a ruler, which makes the scale tilt on that side. It can be concluded that solid has mass which makes the scale tilt downward.
(b) Liquid : A glass full of water is placed on one side of a ruler, which makes the scale tilt on that side. It can be concluded that liquid has mass which makes the scale tilt downward.
(c) Gases : An inflated balloon filled with gas is placed on one side of a ruler, which makes the scale tilt on that side. It can be concluded that gases have mass which makes the scale tilt downward.
Question 4. A measuring cylinder is filled with water to a particular mark. A piece of solid is immersed inside the measuring cylinder. State why the level of water in the measuring cylinder will rise up. If the solid is removed, what will be the new level of the water in the measuring cylinder. Give a reason for your answer.
Answer 4: When the solid is immersed in the cylinder filled with water, the water level rises up:

When the solid is removed, the water level will fall to as it was before the solid is immersed.
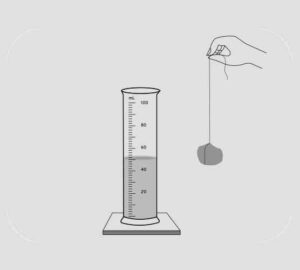
This experiment shows that when a solid is immersed it pushes water upwards by occupying the space of water. It can be concluded that all solids occupy space.
Question 5. A glass beaker is half filled with water and an empty glass tumbler is inverted & lowered inside the glass beaker. State your observations on tilting the tumbler below the level of the water in the glass beaker. Give a reason for your answer.
Answer 5: (1) The empty glass tumbler contains air which is inverted and lowered inside the glass beaker filled with water.
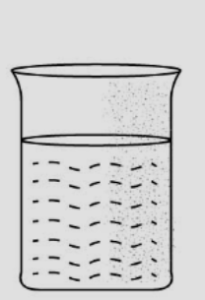
(2) When this empty tumbler is tilted, the air inside the tumbler comes out and bubbles of air are seen. The air inside the tumbler was occupying space inside the beaker.
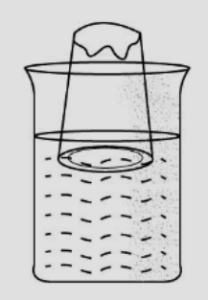
(3) This proves that gases occupy space

Question 6. Differentiate the general properties of solids, liquids and gases in the form of a table — with reference to
(a) Mass
(b) Space
(c) Volume
(d) Shape
(e) Compressibility
(f) Density
(g) Free surfaces
(h) Diffusion
Answer 6:
| Characteristics | Solids | Liquids | Gas |
|---|---|---|---|
| Mass | Have mass | Have mass | Have mass |
| Space | Occupies space | Occupies space | Occupies space |
| Volume | A definite volume | A definite volume | No definite volume |
| Shape | A definite shape | No definite shape | No definite shape |
| Compressibility | No compressibility | Slight Compressibility | High compressibility |
| Density | High Density | Less density | Least density |
| Free Surface | Any number of free surfaces | One upper free surface | No free surfaces |
| Diffusion | No diffusibility | Slight diffusibility | High diffusibility |
Question- 7. State in which of the three states of matter-
(a) Are the atoms or particles far apart
(b) The space between the particles is minimum.
(c) The force of attraction between the particles is very weak.
(d) The movement of the particles are neither about their own positions nor in any random direction.
Answer 7:
(a) Gas
(b) Solid
(c) Gas
(d) Gas
Question-8 Give a reason why –
(a) Solids have a definite volume & a definite shape.
(b) Liquids have a definite volume but no definite shape.
(c) Gases have no definite volume and no definite shape.
Answer 8: (a) Solids have definite shape and definite volume because the molecules in solid are closely packed and in fixed positions. The molecules can vibrate but do not move around which keeps the shape and volume definite.
(b) Liquids have no definite shape but definite volume because the molecules in liquid are not so closely packed and they have space between them. The molecules can move around and the forces of attraction between molecules is less as compared to solids, so the liquid takes the shape of the container and volume is definite.
(c) Gases do not have a definite shape or volume because the molecules in gases are very loosely packed, they have large intermolecular spaces and hence they move around. The force of attraction between molecules is also very less, as a result gases acquire any shape or any volume.
Question 9. Particles of matter possess energy due to their random motion. Compare the particles in a solid, -liquid & in a gas with reference to the amount of kinetic energy possessed by each.
Answer 9: Energy possessed by particles due to motion is kinetic energy. Particles of solid cannot move around because they are closely packed, hence they have least kinetic energy. Particles of liquid are less closely packed, so they move around and hence have large kinetic energy.
Question 10. Describe simple experiments to show that –
(a) particles of matter have inter molecular attraction
(b) particles of matter are closely packed in solids and less in liquids.
Answer 10: (a) To show – Particles of matter have intermolecular attraction.
Experiment – Mercury globules are placed in a petri dish and kept apart. The petri shakes slowly and mercury globules come together to for a big globule. The formation of big globules shows that forces of attraction exist between the molecule

(b) To show – Particles of matter closely packed in solids and less in liquids.
Experiment – A measuring cylinder with 100ml of water is taken and solid sugar crystals are added to it. The solution is stirred carefully to form a sugar solution.
It is observed that volume of water does not change which shows that sugar fills up the intermolecular spaces between water molecules. Hence the water level does not change proving that the intermolecular spaces are more in liquids as compared to solids.
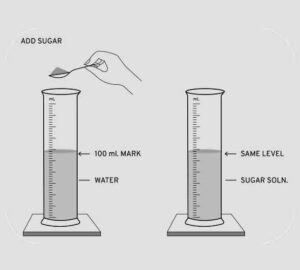
Question 11. A crystal of iodine is placed in a closed flat bottom flask & heated. State how you would conclude from the observations seen, that inter particle space is minimum in solids and maximum in gases.
Answer 11: A crystal of iodine is heated in a closed flask and occupies only the space at the bottom of the flask. After heating, the crystal of iodine converts into vapour and fills the whole flask with iodine vapours.
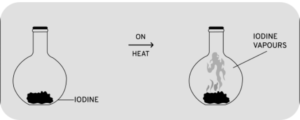
This shows that particles of solid are closely packed and occupy less space while particles of gases are loosely packed and occupy the complete space available.
Question 12. Explain the term ‘inter conversion of matter’ – with ice as a starting material. State the term which refers to the conversion of a substance on heating from.
(a) solid state to liquid state
(b) liquid state to vapour state
(c) vapour state to liquid state
(d) liquid state to solid state.
Answer 12: When the state of matter changes to the other and then back to the original state without changing chemical composition, it is call ‘inter conversion of matter’. The terms which refers to the conversion of a substance are :
(a) Melting
(b) Vaporisation
(c) Liquefaction
(d) Solidification
Question 13. Give a reason why solids and liquids co-exist at their melting points.
Answer 13: A substance is in solid state before melting and in liquid state after melting. The point at which the solid turns liquid is melting point and they coexist at this point.
– : End of Matter and Its Composition Class-7 Dalal Simplified Solutions :–
Return to – Dalal Simplified Chemistry for ICSE Class-7 Solutions
Thanks
Share with your friends.



Don’t display adds
u can disable aid on your device personally
It is very useful and very knowledgeable thank you
thanks for positive feedback
Thank you
ok
Thank you soooooo much. Keep it up .
It is so very useful for studes
It is very useful and easy to study!!
thanks