Physical and Chemical Changes Class-7 Dalal Simplified ICSE Chemistry Solutions Chapter-2 Dr Viraf J Dalal Middle School Allied Publishers Solutions. Chapter-2. We Provide Step by Step Solutions of Exercise/Lesson -2 Questions and Answers of Dr Viraf J Dalal Middle School Chemistry Allied Publishers. Visit official Website CISCE for detail information about ICSE Board Class-7.
Physical and Chemical Changes Class-7 Dalal Simplified ICSE Chemistry Solutions Chapter-2
| Board | ICSE |
| Class | 7th |
| Subject | Chemistry |
| Book Name | Dalal New Simplified |
| Chapter-2 | Physical and Chemical Changes |
| Unit-1 | Physical and Chemical Changes |
| Topic | Solution of exercise questions |
| Session | 2023-24 |
Exercise – 1
Physical and Chemical Changes Class-7 Dalal Simplified ICSE Chemistry Solutions Chapter-2
Question 1. Change is the law of nature and occur in our everyday life, at all times and in all places. Differentiate between the following changes with a suitable example.
-
Desirable and undesirable change
-
Periodic and non-periodic change
-
Slow and fast change
-
Natural and man-made change
-
Reversible and irreversible change.
Answer 1:
(a) Desirable and undesirable change
| Desirable Change | Undesirable change |
|---|---|
| Changes that take place naturally or artificially and produce desirable changes. | Changes that take place naturally or artificially and produce undesirable changes. |
| Exa- ripen of fruit, Rainfall on crops – milk to curd – Growth of plants., | Exa- breaking of goods, Rotting of fruits – Falling of trees – storm , floods |
(b) Periodic and non-periodic change
| Periodic Change | Non-periodic change |
|---|---|
| Changes that take place at regular intervals of time . | Changes which take place irregularly |
| Ex- Change of seasons – Day turning into night | Ex -Rusting of iron ,storm , Thunder and lightning |
(c) Slow and fast change.
| Slow Changes | Fast Changes |
|---|---|
| Changes that take place very slowly, . | Changes that take place quickly . |
| Ex – Growth of trees – rusting – soil formation | Ex – Burning wood – Bursting of crackers – chemical reaction |
(d) Natural and man-made change
| Natural Changes | Man-made Changes |
|---|---|
| Changes that take place naturally and not artificially are natural changes. | Changes that do not take place naturally but are artificial and man-made are man-made changes. |
| Ex- Growth of living | Ex – Fuel to gas – Burning candle |
(e) Reversible and irreversible change.
| Reversible Changes | Irreversible Changes |
|---|---|
| Changes which are not permanent and can be reversed by another change are reversible changes. | Changes which are permanent and cannot be reversed by any change. |
| Ex – Melting of ice | Ex – ripening of fruit |
Question 2. Differentiate between a physical change and a chemical change with reference to –
-
Nature of change i.e. temporary and reversible or permanent and irreversible.
-
Formation of products.
-
Energy change taking place during the respective change.
Answer 2:
| Physical change | Chemical change |
|---|---|
| (a) This change is temporary and reversible. | (a) These changes are permanent and irreversible. |
| (b) There is no formation of new products. | (b) There is formation of new products. |
| (c) There is no energy change in these changes. | (c) Energy is either absorbed or evolved in these changes. |
| Ex – evaporation of water | Ex – ripening of fruit |
Question 3. Give three reasons why melting of wax, is considered a physical change while burning of a candle, a chemical change.
Answer 3: (i) melting of wax, is considered a physical change
Melting of wax is temporary and wax can settle again on cooling and turns into a solid. Hence, the change is only physical and the state of matter changes.
(ii) burning of a candle, a chemical change
Burning of the candle is permanent because once it is burnt it cannot be converted into the candle. A new product is also formed with a composition different from candle. Hence it is a chemical change.
Question 4. State the observations seen, when milk in a dish is kept aside for a few hours or more. Is the change which occurs – a physical change or a chemical change. Give reasons.
Answer 4: When milk is kept aside in a dish for a few hours or more, it is observed that milk turns into curd. This change is chemical as it is permanent and cannot be reversed. A new product i.e. curd is also formed.
Question 5. State what is meant by the term – inter conversion of matter. Is inter conversion of matter a physical change or a chemical change.
Answer 5: The change of state of matter from one state to another state and back to the original state of matter is known as inter-conversion of matter. This change is physical because it is not permanent and reversible.
Question 6.Ice kept in a beaker, slowly melts and turns into water. The water in the beaker on solidification Le. freezing turns back to ice. Give four reasons why the change from ice to water and water back to ice is considered a physical change.
Answer 6: Both Melting of ice and freezing of water are physical changes because:
- These change are temporary
- They are reversible i.e. ice turns into water and water again turns into ice.
- no new products formed
- The composition and properties of water are not altered.
Question 7. Explain the term – ‘sublimation’. Is sublimation of naphthalene – a physical or a chemical change. Give reasons.
Answer 7: The conversion of a solid directly to gaseous form and on cooling back to the solid-state is sublimation.
Sublimation of naphthalene is a physical change because the state of matter changes.
Question 8. Ammonium chloride is also a sublimable solid. Give a reason why sublimation of ammonium chloride involves a physical and a chemical change.
Answer 8: Sublimation of ammonium chloride – [NH4C1] On heating, this sublimes and dissociates into ammonia [NH3] and hydrochloric acid HCl, and recombine on cooling to obtain – NH4C1
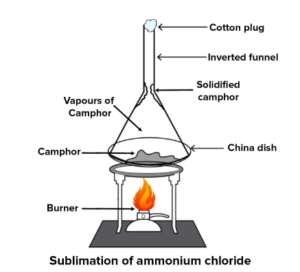
Dissociation – is a chemical change. Hence, sublimation of such substances involves both – a physical and a chemical change.
Question 9. State why addition of sodium chloride to water is considered a physical change, while addition of dilute sulphuric acid to zinc is considered a chemical change.
Answer 9: When water is added to sodium chloride, there is no new product formed, hence it is a physical change.
When dilute sulphuric acid is added to zinc, a new product zinc sulphate is formed, hence it is a chemical change.
Question 10. Photosynthesis is a natural process by which green plants manufacture food in the presence of sunlight.
-
Are any new products formed during the above process.
-
Can the change be reversed or is it irreversible
-
State the conclusions which can be drawn, to represent photosynthesis as a physical or a chemical change.
Answer 10:
- The change is permanent and new products are formed like glucose and oxygen.
- The change is irreversible.
- It is a chemical change because carbon dioxide and water cannot be obtained back from glucose and oxygen.
Question 11. Give reasons why – separation of mixtures e.g. iron from a mixture of iron and sulphur is a physical change, but heating a mixture of iron and sulphur is considered a chemical change.
Answer 11: Why separation of iron from a mixture of iron and sulphur is a physical change:
- The properties of iron and sulphur do not change when they are separated.
- There is no formation of a new product in this change.
- There is no change in the heat energy level of the mixture.
- The change is temporary and reversible as iron and sulphur can be mixed again.
Why heating a mixture of iron and sulphur is a chemical change:
- There are changes in properties of both on heating, they lose their magnetic power.
- A new substance, iron sulphide is formed.
- The heat energy is released when the iron and sulphur mixture is heated. The test tube becomes red hot.
- The change is permanent, which means it cannot be reversed.
Question 12. Explain in brief the involvement of energy in – physical and chemical changes.
Answer 12: Involvement of energy during physical / chemical changes:
In physical changes, energy is used to change the state of matter.
– : End of Physical and Chemical Changes Class-7 Dalal Simplified Solutions :–
Return to – Dalal Simplified Chemistry for ICSE Class-7 Solutions
Thanks
Share with your friends.



In Question number 5 Name the following the last ans The type of change physical or chemical involved – ‘ when a substance undergoes a change in state ,colour or size ‘. The ans should be chemical change but in this pdf it is given physical change when I answered my teacher the teacher told the physical change is wrong . So I want you to fix it up . Thank you 😊
thanks for catching mistake
it is now updated