Goyal Brothers Class-10 Mole Concept and Stoichiometry Ch-5. Step by Step Solutions of Practice Problems , Exercise and Objective Type Questions of Goyal Brothers Prakashan Chapter-5 Mole Concept and Stoichiometry for ICSE Class 10 Chemistry.
Practice Problems Numerical based on Volume to Volume relation, Volume to Weight relation and Weight to Weight relation of mole concept and Stoichiometry for ICSE Class-10 Goyal Brother Prakashan Chemistry. Visit official Website CISCE for detail information about ICSE Board Class-10 Chemistry .
Goyal Brothers Class-10 Mole Concept and Stoichiometry Ch-5
-: Select Topics :-
Objective Type Questions
Practice Problems Mole Concept and Stoichiometry Ch-5 ICSE Class-10
(Numerical Problems Based on Gay Lussac’s Law )
Page-71 Practice Problems-1
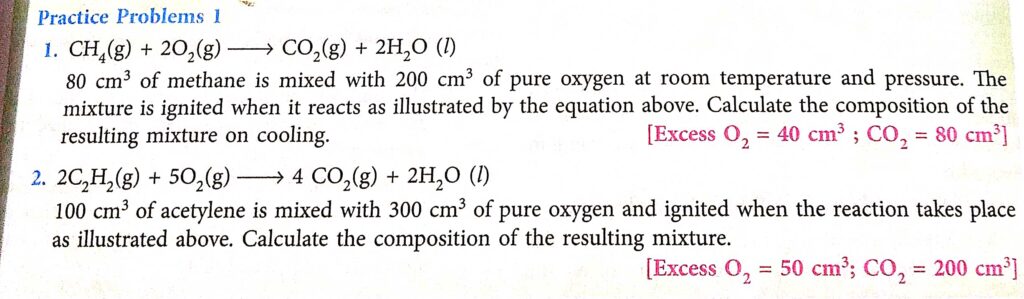
Answer
(1)
Thus, gaseous composition after reaction is:
Excess oxygen = 200 – 160 = 40 cm3
CO2 = 80 cm3
Water = negligible
(2)
2 C₂H₂ + 5 O₂ ======> 4 CO₂ + 2 H₂O
100 cm³ of acetylene is mixed with 300 cm³ of pure oxygen and ignited ,
According to the equation :
2 volumes of C₂H₂ + 5 volumes of O₂ ===> 4 volumes of CO₂ + 2 volumes of H₂O
Now it is given that C₂H₅ has 100 cm³ volume .
So 2 volumes = 100 cm³ .
⇒ 1 volume = 100 cm³/2
⇒ 1 volume = 50 cm³
300 cm³ is ignited .
Given 5 volumes of oxygen react .
⇒ 5 × 50 cm³ react .
⇒ 250 cm³ react .
Excess oxygen can be calculated by subtracting the amount of reacted oxygen by total volume of oxygen ignited .
Unused Oxygen = 300 cm³ – 250 cm³
⇒ Unused oxygen = 50 cm³ .
Total volume of the resulting mixture :
4 volumes of CO₂ + 2 volumes of H₂O
⇒ 4 × 50 cm³ + Nil
⇒ 200 cm³ + Nil
200 cm³
Page-72 Practice Problems-2

Answer
(1)
Given react ion is :
N2 + O2 → 2NO
According to Gay -Lussac’s law in the above reaction 1 volume of nitrogen combines with 1 volume of oxygen to produce 2 volumes of nitric oxide.
i .e. N2 + O2 → 2NO
1 vol. 1 vol. 2 vol.
The volume of nitric oxide produced is = 1400cm3.
Let the volumes of nitrogen and oxygen gases be = x
Then, N2 + O2 → 2NO
x x 1400cm3
So, x + x = 1400
2x = 1400
x= 1400/ 2 = 700cm3
Hence the volumes of reacting gases i .e. nitrogen and oxygen is 700 cm3 each.
(2)
2C2H2(g) + 5O2(g) → 4CO2(g)+ 2H2O(g)
4 V CO2 is collected with 2 V C2H2
So, 200cm3 CO2 will be collected with = 100cm3 C2H2
Similarly, 4V of CO2 is produced by 5 V of O2
So, 200cm3 CO2 will be produced by = 250 ml of O2
Numerical Problems Based on Avogadro’s Law
(Class-10 Mole Concept and Stoichiometry Ch-5)
Page-79 Practice Problems-1
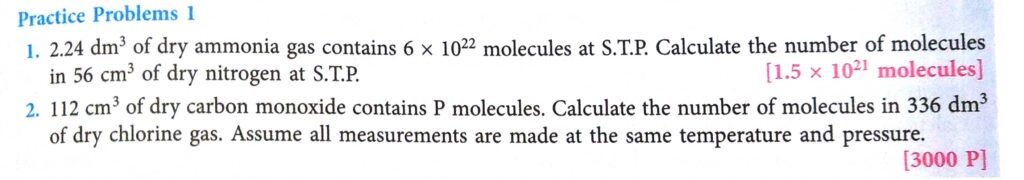
Answer
(1)
At STP, gas occupies volume.
So, gas have
Number of moles of dry Nitrogen at STP
Number of molecules of dry Nitrogen
Avogardo Number
So, Nitrogen have or
(2)
According to Avogadro’s law,
If 112cc of dry CO contains P molecules,then 112 cc of dry Cleaners will also contain P molecules.
1dm3=1000 cc
so 336 dm3 =1000 x 336 cm3
Applying unitary method,
112cc => P molecules
336000cc => 336000 x P/ 112 = 3000P.
Page-79 Practice Problems-2

Answer
(1)
5000 molecules of H2 =V
By Avogadro’s law
5000 molecules of N2 =V
2.5 *108 ÷5000=5 x 10⁴ V
(2)
6* 1022 present in 2.24 dm3 of Cl
By Avogadro’s law
3 *1019 molecule = 3 *1019 (2.24/6* 1022 )
1.12*10-3 dm3
Numerical Problems Based on Molecular Weight
(Class-10 Mole Concept and Stoichiometry Ch-5)
Page-80 Practice Problems-1

Answer
(i)
Al+3 (O+ H)
=27+3(16+1)
=27+51
=78 amu
(ii)
= 2 K+ 2 Cr+ 7 O
= 2x 39 + 2 x 52 + 7 x 16
= 78 + 104 + 112
=294 amu
(iii)
The molar mass of ammonium nitrate= sum of the molar mass of all its constituents in the correct proportion.
Molecular weight of nitrogen = 14.
Molecular weight of hydrogen= 1.
The molecular weight of oxygen= 16
The molecular weight of ammonium nitrate= 2 * weight of nitrogen atom + 4 * weight of hydrogen atom + 3 * weight of an oxygen atom
= 28 + 4. + 48
= 80 amu
Page-80 Practice Problems-2
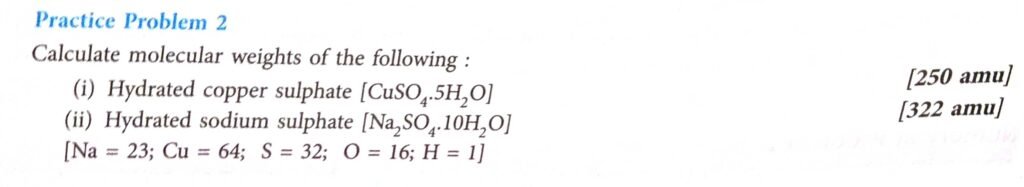
Answer
(i)
MM of Cu + S +4 O + 5 (2H + O)
=64+32+ 4* 16 + 5(2*1 +16)
=64+32+64+90
=250amu
(ii)
MM of 2 Na + S +4 O + 10 (2H + O)
= 2 *23 +32 + 4* 16 + 10 (2*1 +16)
= 46+32 +64+ 180
= 322 amu
Numerical Problems Based on Avogadro’s Number
(Goyal Brothers Class-10 Mole Concept and Stoichiometry for ICSE Chemistry Ch-5)
Page-80 Practice Problems-1

Answer
(1)
Number of molecules in 12.8g of sulphur dioxide gas.
Molecular mass of SO2 = 64a.m.u .
So, 64g = 1 mole
12.8g = 12.8/ 64 = 0.2 mole
Now 1 mole of SO2 contains = 6 x 1023molecules
0 .2 mole of SO2 contains = 0 .2 x 6 x 1023
= 1.2 x 1023molecules
(2)
71g of chlorine has 6×10^23 molecules
7.1g has 0.1×6×10^23 molecules
=6×10^22
Page-81 Practice Problems-2

Answer
(1)
We know
One mole contains 6×10^23 molecules
Weight of one mole of nitrogen gas= 28gm
Weight of 2.8×10^24 molecules
= (2.8×10^24/6×10^23) * 28
= 28×28/6
= 130.67 gm
(2)
gram molecular mass of Coso4 is =159
6*x 1023molecules weight = 159 g
1 molecule weight is = 159/ 6 *x 1023
2*x 1023molecules weight is = (159 * 2*x 1023)/ (6 *x 1023 )
=5.33 gm
Page-81 Practice Problems-3

Answer
(1)
O2 is a diatomic molecule.
The mass of an oxygen atom = 16 amu.
∴ Mass of O2 molecule = 2 × 16 = 32 amu.
Mass of one molecule of oxygen
⇒ 32/ Avogadro constant ( 6.0 × 1023)
⇒ 5.33 × 10-23g.
(2)
O3 is a triatomic molecule.
The mass of an oxygen atom = 16 amu.
∴ Mass of O3 molecule = 3 × 16 = 48 amu.
Mass of one molecule of oxygen
⇒ 48/ Avogadro constant ( 6.0 × 1023)
⇒ 8 × 10-23g.
Numerical Based on Gram Molecular Volume Mole Concept and Vapour Density
(Goyal Brothers Class-10 Mole Concept and Stoichiometry for ICSE Chemistry Ch-5)
Page -82 Practice Problem -1

Answer
(1)
Mass of one mole of nitrogen dioxide = 14 + 16 + 16 = 46g
No. of moles in 0.23g on NO2 = 0.005
volume occupied by 1 mole = 22.4 L
volume occupied by 0.005 mole = 22.4*0.005
=0.112 L =0.112 dm3
0.112 L OR 112 mL:
(2)
Mass of one mole of Butane = 4*12+10*1 = 58
No. of moles in 2.9 g butane = 2.9/58
volume occupied by 1 mole = 22.4 L
volume occupied by 2.9/58 mole = (22.4* 2.9)/58
=1.12 dm3
Practice Problem -2 Page -82

Answer
(1)
Molecular mass of co2 = 44 g
22.4 L = 22.4 dm3 = 44 g
hence 1 L = 44/22.4 g
therefore 5.6 dm3 = 5.6 * 44/22.4 g
= 11 g
(2)
Molecular mass of butane = 58 g
22.4 L = 22.4 dm3 = 58 g
hence 1 L = 58/22.4 g
therefore 7.84 dm3 = 7.84 * 58/22.4 g
= 20.3
Page -82 Practice Problem -3

Answer
(1)
(i)
MM of lead nitrate = 207+14+16*3
=207+14+48
=269 g
Mole = Mass /MM
Mole= 6.62/269=0.024 mole ans
(ii)
1 mole =6 × 1023molecule
0.024 mole = 0.024 x 6 × 1023.
1.2 × 1022molecule.
(2)
(i)
mole = mass/MM
mole=2/100
=0.02 moles
(ii)
1 mole =6 × 1023molecule
=0.02 mole = 0.02 x 6 × 1023.
=1.2 × 1022molecule.
Page-83 Practice Problem -4

Answer
(1)
(i)
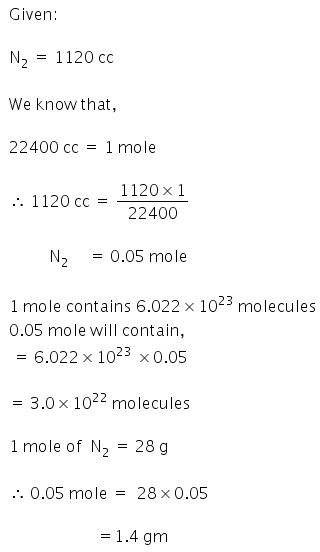
(ii)
(2)
(i)
…………….
(ii)
Volume of carbon dioxide gas = 44.8 L
Since at STP ; 22.4 L = 1 mole of gas
=2 moles of gas.
(iii)
………………..
Practice Problem -5 Page -83

Answer
(1)
Under similar conditions same volume of two gases have equal no of moles so, number of moles of X = number of moles of hydrogen
Under similar conditions same volume of two gases have equal no of moles so, number of moles of X = number of moles of hydrogen

Answer :
(1)
MM =2 x VD =2 x 32 = 64
(i) number of moles= 0.56/22.4 =0.025
(ii) Weight in grams
1 mole weight = 64
so 0.025 mole weight = 0.025 x 64 = 1.6 gm
(iii) number of molecules
1 mole = 6.023 x 1023
0.025 mole = 0.025 x 6.023 x 1023
1.505 x 1022
(2)
MM =2 x VD =2 x 8.5 = 17 gm
(i) number of moles= 8.96/22.4 =0.4 mole
(ii) Weight in grams
1 mole weight = 17
so 0.4 mole weight = 0.4 x 17 = 6.8 gm
(iii) number of molecules
1 mole = 6.023 x 1023
0.4 mole = 0.4 x 6.023 x 1023
2.4 x 1022
Exercise-1 Goyal Brothers Prakashan Class-10 Mole Concept and Stoichiometry Ch-5
Page-84
Question 1. State “Gay Lussac’s Law” of combining gas. Support your answer by two examples.
Answer :
This law, formulated by Gay Lussac, states that, “the ratio between the volumes of gaseous reactants and products can be expressed in simple whole numbers.
For example, in the following reaction, the ratio of volumes of hydrogen, chlorine, and hydrogen chloride is 1:1:2 (a simple ratio):
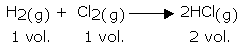
Question 2. State “Avogadro’s Law” Explain the law for reaction between nitrogen and hydrogen, when they react to form ammonia gas.
Answer :
Avogadro’s law states that equal volumes of different gases contain an equal number of molecules. This applies when the temperature and pressure stay the same.
Avogadro’s law can be used to calculate the volumes of gases involved in reactions.
Question 3. What do you understand by the term “atomic mass unit”?
Answer :
a unit of mass used to express atomic and molecular weights, equal to one twelfth of the mass of an atom of carbon-12.
Question 4. Define relative atomic mass of an element.
Answer :
A relative atomic mass (also called atomic weight; symbol: Ar) is a measure of how heavy atoms are. It is the ratio of the average mass per atom of an element from a given sample to 1/12 the mass of a carbon-12 atom.[1][2] In other words, a relative atomic mass tells you the number of times an average atom of an element from a given sample is heavier than one-twelfth of an atom of carbon-12
Question 5. What is gram atomic mass?” How does gram atomic mass differ from relative atomic mass of an element ?
Answer :
Gram atomic mass(weight) of an element is the mass of Avogadro number ( 6.023 x 1023 ) of atoms of that elements in grams.”
Ex –
mass of 1 atom of oxygen = 16 amu = 16 x 1.66 x 10-24 gm
Mass of 6.023 x 1023 atoms of oxygen= 16 x 6.023 x 1023 x 1.66 x 10-24 = 16 gm.
Hence, Gram atomic weight of oxygen = 16 gm.
Question 6. Define relative molecular mass of a substance. How does it differ from gram molecular mass.
Answer :
Expression of molar mass is grams per mole. It can also be expressed as kilogram per mole. Molecular mass is expressed in atomic mass units
Molecules are substances formed due to combinations of the same or different atoms in different ratios. These molecules attached to each other to form compounds. Besides, the molar mass and molecular weight are two physical properties of substances.
Question 7. Define gram molecular volume. State its experimental value.
Answer :
gram–molecular volume, the volume occupied by one gram–molecular weight of any substance at STP. Its value at STP is 22.4 liter / 22400 ml/ 22.4 dm3 / 22400 cm3
Question 8. Define Avogadro’s number. State its value.
Answer :
Avogadro’s number, number of units in one mole of any substance (defined as its molecular weight in grams), equal to 6.02214076 × 1023. The units may be electrons, atoms, ions, or molecules, depending on the nature of the substance and the character of the reaction (if any)
Question 9. What do you understand by the term “mole”? How many elementary units are in one mole of a substance
Answer :
A mole is defined as 6.02214076 × 1023 of some chemical unit, be it atoms, molecules, ions, or others. The mole is a convenient unit to use because of the great number of atoms, molecules, or others in any substance
Question 10. Fill in the blank spaces:
(i) Weight of a substance (in grams) = Number of moles X …
(ii) Volume of a gas at S.T.P=… .. x 22.4 dm³ at S.T.P
Answer :
(i) Weight of a substance (in grams) = Number of moles X …Molecular mass
(ii) Volume of a gas at S.T.P=…No of Mole .. x 22.4 dm³ at S.T.P
Question 11. State four applications of Avogadro’s Law.
Answer :
four applications of Avogadro’s Law
(i) It determines the molecule formula of a gas.
(ii) It determines atomicity of gases.
(iii) It explains Gay-Lussac’s law of combining volumes.
(iv) It establishes the relation between molecular weight and vapour density of a gas
Question 12.
(i) What do you understand by the term atomicity?
(ii) What is the atomicity of the following elements (1) helium (2) nitrogen (3) ozone (4) phosphorus (5) sulphur?
Answer :
(i) Atomicity is defined as the total number of atoms that constitute a molecule. For example, each molecule of oxygen (O2) is composed of two oxygen atoms. So atomicity of oxygen is 2.In older contexts, the term atomicity is sometimes used in the same sense as valency
(ii) the atomicity of the following elements
(1) helium–1
(2) nitrogen –2
(3) ozone—3
(4) phosphorus—5
(5) sulphur—8
Question 13.
(i) What do you understand by the term vapour density?
(ii) How is vapour density of a substance related to its gram molecular mass?
Answer :
(i) Vapour density is the density of a vapour in relation to that of hydrogen. It may be defined as mass of a certain volume of a substance divided by mass of same volume of hydrogen.
(ii) The vapour density of a substance related to its gram molecular mass?
molar mass = 2 × vapour density
Part-2 Stoichiometry Goyal Brothers Prakashan ICSE Class-10
Numerical Based on Percentage Composition
Practice Problems-1 Page- 85
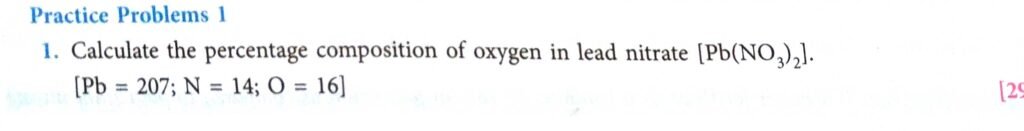

Answer
(1)
Pb + (N)2 + (O)6
207 + 2 x 14 + 6 x 16 = 331.
So, the molecular mass of Pb(NO3)2 = 331.
331 by weight of Pb(NO3)2 contain 96 parts by weight of oxygen .
100 parts will contain = 96 x 100 / 331 = 29 ‰
So, the percentage composition of oxygen in lead nitrate is 29‰
(2)
Atomic mass of calcium (Ca) = 40
Atomic mass of phosphorus (P) = 31.
Atomic mass of oxygen (O) = 16.
Now, molecular mass of calcium phosphate = 40 (3) + (31+64)2 = 120 + 95(2) = 120+190 = 310 g/mol.
Percentage of Ca in Ca3(PO4)2 = 40(3)/310 ×100 = 120/310 ×100 = 38.7….%
(3)
| . Write the formula for the given compound. | Na2CO3 •10H2O |
| 2. Calculate the molar mass of each part of the compound separately. Also, the number in front of the water molecule must be distributed and multiplied by the subscript of both the hydrogen and the oxygen in the water molecule. (Alternative: Water has a gram formula mass of 18.0 g/mol. Rather than go through the aforementioned process, multiply the coefficient in front of the water molecule by 18.0. In this problem there are 10 water molecules. 10 x 18.0 = 180.0, the same result as on the right.) | Na: 2 x 23.0 = 46.0 C: 1 x 12.0 = 12.0 O: 3 x 16.0 = 48.0 106.0H: 20 x 1.0 = 20.0 O: 10 x 16.0 = 160.0 180.0 |
| 3. Add the totals for each part together to find the molecular formula mass. | 106.0 + 180.0 = 286.0 |
| 4. Divide each part’s mass by the molecular formula mass. | Na2CO3: 106.0 ÷ 286.0 = 0.371 10H2O: 180.0 ÷ 286.0 = 0.629 |
| 5. Multiply each result by 100 in order to get a percentage. | Na2CO3: 0.371 x 100 = 37.1% 10H2O: 0.629 x 100 = 62.9% |
(4)
The formula mass of ferrous sulphate
So % of water of crystallisation %
Practice Problems-2 Page- 86

Answer
(1)
50 x 60/100 =30 gm
Molecular mass of KClO3 = 39+35.5+ 16 x 3
=122.5
% of O2 = (48 x 100 /122.5)
………….39.18……..
now
39.18 x 60/100 = 23.5%
(2)
Molecular weight of

Answer
(1)
The mass of N is 28g.
Percent composition of N is = 28*100/80 =35%
The mass of N is 42g.
Percent composition of N is = 42*100/149 =28.19%
hence ammonium nitrate is better
(2)
Given Urea= NH2- CO- NH2 And we know that Total mass of urea = 60 Mass of nitrogen = 14 Two molecules of nitrogen= 2*14= 28 Percentage of nitrogen = Mass of Nitrogen/(Urea ) x 100 = 28/60 x 100 = 46.6% Hence Percentage of nitrogen in urea is 46%
You can write the percent composition of nitrogen in ammonium sulfate by dividing the molar mass of the two nitrogen atoms by the molar mass of the compound, and multiplying the result by 100. This means that every 100g of ammonium sulfate contain 21.2 g of nitrogen
hence urea is better
Numerical Problems Based on Empirical Formula and Molecular Formula
(Goyal Brothers Class-10 Mole Concept and Stoichiometry for ICSE Chemistry Ch-5)
Practice Problems-1 Page- 89

Answer
(1)
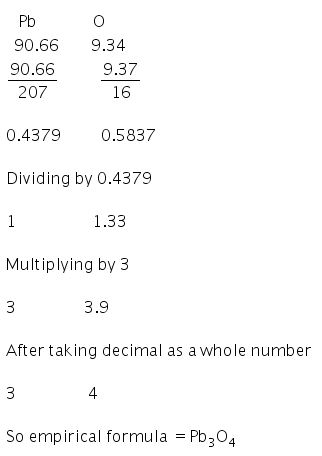
atomic mass of Pb = 207 g/mol
atomic mass of N = 14 g/mol
atomic mass of O = 16 g/mol
percentage composition:
Pb = 62.5% , N = 8.5% and O = 29%
Now finding percentage mass ratio,
Pb ⇒ 62.5/207 = 0.3063
N ⇒ 8.5/14 = 0.6071
O ⇒ 29/16 = 1.8125
finding simple ratio of given elements,
Pb : N : O : : 0.3063 : 0.6071 : 1.8125
⇒Pb : N : O : : 0.3 : 0.6 : 1.8
⇒ Pb : N : O : : 1 : 2 : 6
hence, empirical formula of compound is
(3)
Let amount of organic =
=Al2 (SO4)3
(4)
mole value of Na= 16.10/23=0.7
mole value of C= 4.20/12=0.35
mole value of O= 16.77/16= 1.08
simple mole ratio of Na= 0.7/0.35=2
simple molar ratio of C=0.35/0.35=1
simple mole ratio of O= 1.08/0.35= 3
so, empirical formula is
Na2CO310H2O
Practice Problem-2 Page-90
Answer
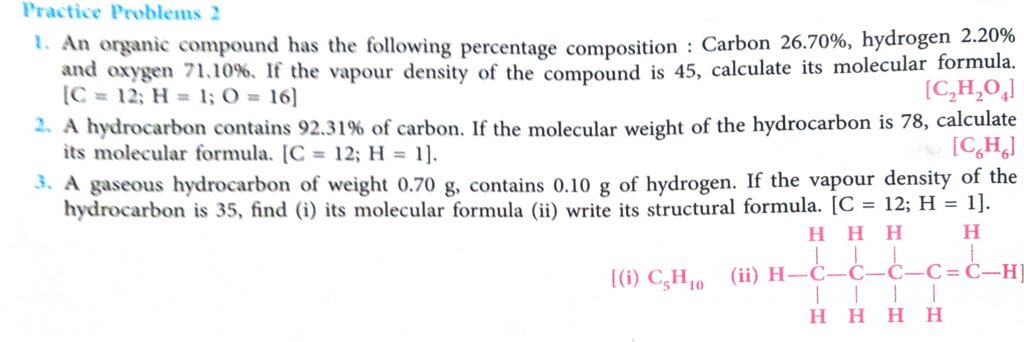
(1)
mole value of C= 26.70 /12 = 2.21
mole value of H= 2.20/1= 2.22
mole value of O= 71.10/16= 4.44
(a) its empirical formula = CHO2
(b) empirical formula mass = 45
Vapour density = 45
So, molecular mass = V.D × 2 = 90
so, molecular formula = C2H2O4
(2)
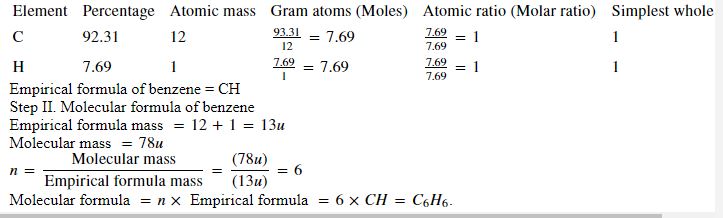
(3)

Practice Problem-3 Page-91

Answer
(1)
VD= Empirical formula
VD= 12+1 x2+ 16=30
Molecular Formula =2 * Empirical formula
= 2( CH2O)
= C2H4O2
(2)
EF = 2 VD =Molecular Formula
12*2 + 1*6 +16 = 46
Empirical formula is same to Molecular Formula
C2H6O
Exercise 2 Goyal Brothers Class-10 Mole Concept and Stoichiometry Ch-5
Page-91
Question 1. What do you understand by the term “molecular formula”?
Answer :
a chemical formula that indicates the kinds of atoms and the number of each kind in a molecule of a compound.
Question 2. What do you understand by the term “empirical formula”?
Answer :
In chemistry, the empirical formula of a chemical compound is the simplest positive integer ratio of atoms present in a compound. .An empirical formula makes no mention of the arrangement or number of atoms
Question 3. What is the empirical formula of the following chemical compounds:
(i) C6H6 (ii) C6H12O6 (iii) CH3COOH, (iv) C2H5OH, (v) C2H4 (vi) C2H2
Answer :
the empirical formula of the following chemical compounds:
(i) C6H6 —CH
(ii) C6H12O6—-CH2O
(iii) CH3COOH,—–CH2O
(iv) C2H5OH,—-C2H6O
(v) C2H4—– CH2
(vi) C2H2——CH
Question 4. What is the difference between molecular formula and empirical formula?
Answer :
Molecular formulas tell you how many atoms of each element are in a compound, and empirical formulas tell you the simplest or most reduced ratio of elements in a compound. If a compound’s molecular formula cannot be reduced any more, then the empirical formula is the same as the molecular formula
Question 5. The molecular weight of a chemical compound is six times its empirical formula weight. If the empirical formula of the compound is CH2, find its molecular formula.
Answer :
empirical mass = CH2 = 12+ 2×1 =14
molecular mass= 6 time of empirical mass
molecular mass= 6 x 14
molecular mass = 84
n = molecular mass/ empirical mass
n= 84/14
n=6
Molecular formula = n (empirical formulas)
Molecular formula = n (CH2)
Molecular formula = 6 (CH2)
Molecular formula = C6H12
A. Problems on Weight – Weight Relationships
( Goyal Brothers Class-10 Mole Concept and Stoichiometry for ICSE Chemistry Ch-5)
Practice Problem-1 Page-92

Answer
(1)
molecular mass of ammonium nitrate =80 g
mass of nitrogen in ammonium nitrate= 28
when ammonium nitrate 80 g then nitrogen is= 28
when ammonium nitrate 1 g then nitrogen is= 28/80
when ammonium nitrate 100*1000 g then nitrogen is= (28*100*1000)/80
=35 000 g = 35 kg
(2)
molecular mass of ferric oxide(Fe2O3) =160 g
mass of iron in ferric oxide(Fe2O3)=56*2 = 112
when ferric oxide(Fe2O3) 160 g then iron mass= 112
when ferric oxide(Fe2O3) is 1 g then iron mass= ( 112) /160
when ferric oxide(Fe2O3) is 60*1000*1000 g then iron mass= (112*60*1000*1000)/160
=5600000 g = 5600 quintol= 56 tonn
Practice Problem-2 Page-93

Answer of Practice Problem-2 Page-93
(1)

Now calculate weight of potassium chloride formed
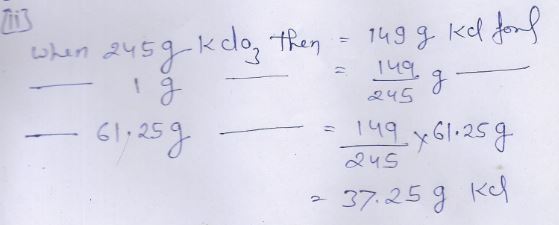
( 2)
(i)
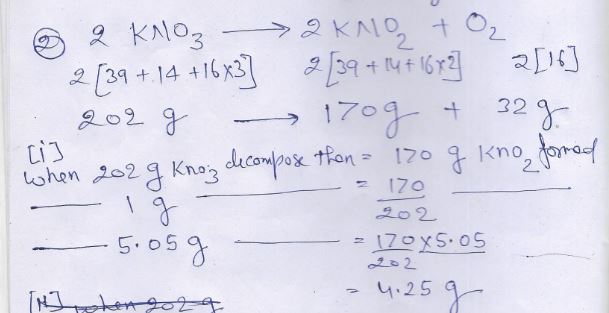
(ii)
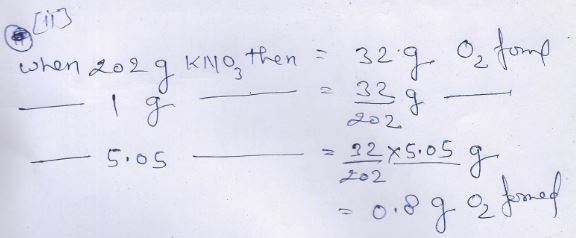
Practice Problem-3 Page-93
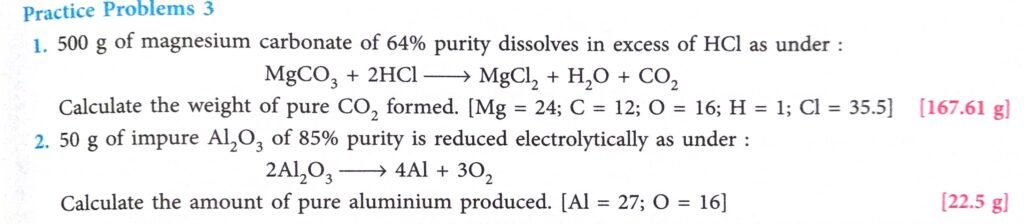
Answer
(1)
Pure MgCO3 mass= 500 x 60/100
=320 gm
Mole of MgCO3 =Mass / MM
= 320/84
=3.81 mole
1 mole MgCO3 form = 1 mole CO2
3.81 mole = 3.81 mole CO2
hence
1 mole CO2 = 44 gm
3.81 mole CO2 =3.81 x 44
= 167.62 gm
(2)

Practice Problem-4 Page-94

Answer
(1)

( 2 )

B- Weight- Volume Relationship
( Goyal Brothers Class-10 Mole Concept and Stoichiometry for ICSE Chemistry Ch-5)
Practice Problem-1 Page-94 , 95

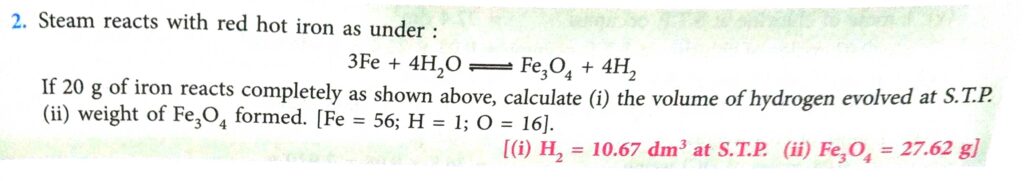
Answer
 (2)
(2)

C. Chemical Calculation Based on Mole Concept
( (Goyal Brothers Class-10 Mole Concept and Stoichiometry for ICSE Chemistry Ch-5 )
Practice Problem-1 Page -95
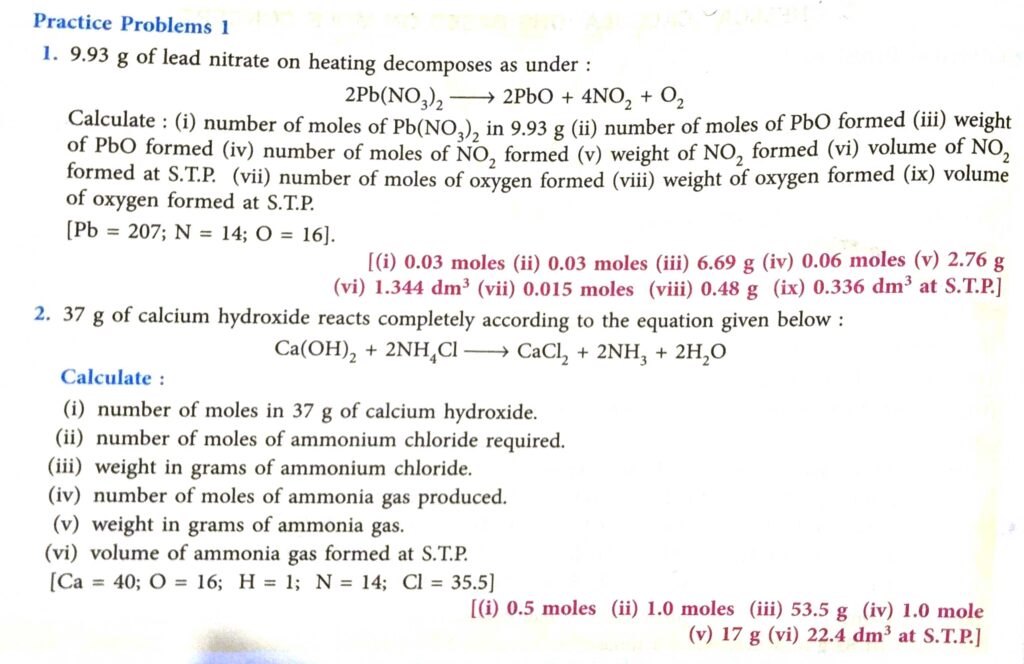
Answer 


(2)

Objective Type Questions
(Goyal Brothers Class-10 Mole Concept and Stoichiometry )
I. Multiple Choice Questions
Choose the correct answer from the options given below :
- ……………..
- …………….
- …………..
- ………..
- ……………………
- …………
- …………
- ……………..
- ………..
- ………………………
- ………………………….
- …………………….
- ………………….
- ……………….
- …………………..
- ……………….
- ………..
- ……………….
- ……………………….
- …………………….
II Fill in the blanks space with the choices given in brackets:
- …………
- ………….
- ……….
- …………
- ………..
- ………….
- …………
- …………
- …………………
- …………………
III Choose from the following list, as what matches the descriptions given blew.
- …………………
- ……………
- ………….
- …………
- ………
- ……….
- ………
- ……….
- ………
- ……………..
–: End of Mole Concept and Stoichiometry Solutions Goyal Brothers :–
Return of : Chemistry Class-10 Goyal Brothers Prakashan
Thanks
Share with your friends


Pls provide mcq and fillups answers also
Pls….