Heat and Energy Concise Class-9 ICSE Physics Selina Publishers Solutions Chapter-6 . Step By Step ICSE Selina Concise Solutions of Chapter-6 Heat and Energy with Exercise-6(A), Exercise-6(B) , Exercise-6(C) and Exercise-6(D) including Numerical and MCQ Questions Solved . Visit official Website CISCE for detail information about ICSE Board Class-9.
| Board | ICSE |
| Publications | Selina Publication |
| Subject | Physics |
| Class | 9th |
| Chapter-6 | Heat and Energy Exe-6(A) |
| Book Name | Concise |
| Topics | Solution of Exe-6(A), MCQ-6(A), Exercise-6(B), MCQ-6(B) Exercise-6(C), MCQ-6(C) and Exercise-6(D), MCQ-6(D) |
| Academic Session | 2021-2022 |
Heat and Energy Concise Class-9 ICSE Physics Selina Publishers
–: Select Topics :–
Exercise-6(A), MCQ-6(A)
Note :- Before Viewing Selina Concise Physics Solutions of Chapter-6 Heat and Energy for ICSE Class-9 Physics. Read the whole chapter carefully and Solved all example of Chapter-6 Heat and Energy for Class-9 Physics.
EXE-6(A) Heat and Energy Selina Concise Physics Solutions
Page 129
Question 1
What is heat? Write its S.I. unit.
Answer 1
Heat is the energy of random motion of molecules constituting the body.
Its S.I. unit is ‘joule’.
Question 2
Two bodies at different temperatures are placed in contact. State the direction in which the heat will flow.
Answer 2
Heat will flow from a hot body (body at a higher temperature) to a cold body (body at a lower temperature)
Question 3.
Name the S.I. unit of heat and how is it related to the unit calorie?
Answer 3
S.I. unit of heat is ‘joule’.
1 joule = 0.24 cal
Question 4
Define temperature and write its S.I. unit.
Answer 4
Temperature is the parameter which tells the thermal state of a body (i.e. the degree of hotness or coldness).
The S.I. unit of temperature is ‘kelvin’
Question 5
Why does a piece of ice feel cool to touch? Explain.
Answer 5
On touching a piece of ice, heat flows from our hand (hot body) to the ice (cold body), and hence, it appears cold.
Question 6
Distinguish between heat and temperature.
Answer 6
Heat is a form of energy obtained due to the random motion of molecules in a substance but temperature is a quantity which decided the direction of flow of heat when two bodies at different temperature are placed in contact. Two quantities having the same amount of heat may differ in temperature.
Question 7
What do you understand by thermal expansion of a substance?
Answer 7
The expansion of a substance on heating is called thermal expansion.
Question 8
Name two substances which expands on heating.
Answer 8
Brass and iron expand on heating.
Question 9
Name two substances which contract on heating.
Answer 9
Water contracts on heating from 0 to 4.
Silver iodide contracts on heating from 80 to 141.
Question 10
What do you mean by the anomalous expansion of water?
Answer 10
The expansion of water when it is cooled from 4 to 0 is known as the anomalous expansion of water.
Question 11
At what temperature is the density of water maximum? State its value.
Answer 11
Density of water is maximum at 4. Its value is 1000 kgm-3.
Question 12
State the volume changes observed when a given mass of water is heated from 0°C to 10 °C . Sketch a temperature-volume graph to show the behaviour.
Answer 12
When a given mass of water is heated from 0°C to 4 °C , it contracts, i.e. its volume decreases.
On heating from 4 °C to 10 °C , it expands, i.e. its volume increases.
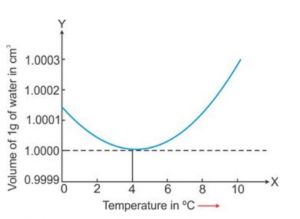
Question 13
Draw a graph to show the variation in density of water with temperature in the range from 0 °C to 10 °C.
Answer 13
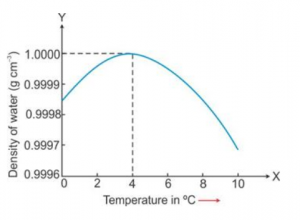
Question 14
A given mass of water is cooled from 10°C to 0°C. State the volume changes you will observe. Represent these changes on a temperature-volume graph.
Answer 14
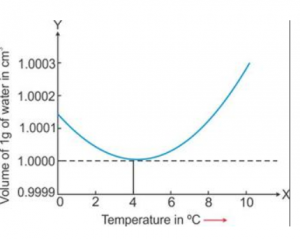
Page 130
Question 15
Describe an experiment to show that water has maximum density at 4°C. What important consequences follow this peculiar property of water? Discuss the importance of this phenomenon in nature.
Answer 15
Hope’s experiment to demonstrate that water has maximum density at 4:
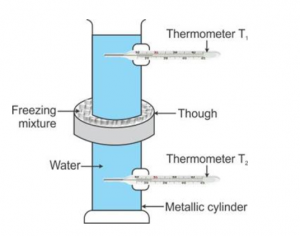
Hope’s apparatus consists of a tall metallic cylinder provided with two side openings P and Q, P near the top and Q near the bottom, fitted with thermometers T1 and T2 in them. The central part of the cylinder is surrounded with a cylindrical trough containing a freezing mixture of ice and salt. The cylinder is fitted with pure water at room temperature.
Observations:
(i) Initially, both thermometers T1 and T2 are at the same temperature.
(ii) First, the temperature recorded by the lower thermometer T2 starts decreasing and finally it becomes steady at 4, while the temperature recorded in the upper thermometer T1 remains almost unchanged during this time.
(iii)
Then, the temperature recorded by the lower thermometer T2 remains constant at 4°C and upper thermometer T1 records a continuous fall in temperature up to 0 °C and then it becomes steady.
Thus, finally, the temperature recorded by the upper thermometer is 0 °C and that by lower thermometer is 4.°C
As the freezing mixture cools water in the central portion of the cylinder, water contracts and its density increases, consequently it sinks to the bottom, thereby causing the reading of the lower thermometer T2 to fall rapidly. The reading of the upper thermometer T1 does not change as the temperature of water in the upper part does not change. This continues till the entire water below the central portion reaches 4°C .
On cooling further below 4 °C , due to anomalous expansion, water of the central portion expands, so its density decreases and hence it rises up. As a result, reading of the upper thermometer T1 falls rapidly to 0 °C and water freezes to form ice at 0 °C near the top. This proves that water has maximum density at 4.°C
This anomalous expansion of water helps in preserving the aquatic life during the very cold weather.
In winters, when the temperature falls, the top layer of water in a pond contracts, becomes denser and sinks to the bottom. A circulation is thus set up until the entire water in the pond reaches its maximum density at 4°C . If the temperature falls further, then the top layer expands and remains on the top till it freezes. Thus, even though the upper layers are frozen, the water near the bottom is at 4 °C and the fishes can survive in it easily.
Question 16
A deep pond of water has its top layer frozen during winter. State the expected temperature of water layer (i) Just in contact with ice and (ii) At the bottom of pond.
Answer 16
(i) Water just in contact with ice is at 0°C.
(ii) Water at the bottom of the pond is at 4°C.
Question 17
Draw a diagram showing the temperature of various layers of water in an ice covered pond.
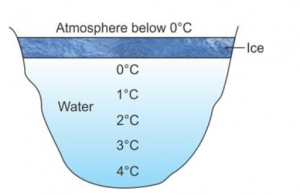
Question 18
Explain the following statements listed below:
(a) Water pipes in colder countries often burst in winter.
(b) In winter, water tanks (or ocean) start freezing from the surface and not from the bottom.
(c) Fishes survive in ponds even when the atmospheric temperature is well below 0°C.
(d) A hollow glass sphere which floats with its entire volume submerged in water at 4°C , sinks when water is heated above 4°C.
(e) A glass bottle completely filled with water and tightly closed at room temperature is likely to burst when kept in the freezer of a refrigerator.
Answer 18
(a) On winter nights, as the atmospheric pressure starts falling below 4 °C , water in the pipe lines expand and exert a great pressure on the pipes, causing them to burst.
(b) In winters, when temperature falls, the surface of water in the tank contracts, becomes denser and sinks to the bottom. A circulation is thus set up until the entire water in the tank reaches its maximum density at 4°C . If the temperature falls further, then the top layer expands and remains on the top till it freezes. Thus, water in a tank starts freezing from the top and not from the bottom.
(c) The anomalous expansion of water
helps preserve aquatic life during very cold weather. When temperature falls, the top layer of water in a pond contracts becomes denser and sinks to the bottom. A circulation is thus set up until water in the pond reaches its maximum density at 4 °C . If the temperature falls further, then the top layer expands and remains on the top till it freezes. Thus, even though the upper layer are frozen, the water near the bottom is at 4 °C and the fishes can survive in it easily.
(d) On heating water above 4 °C the density of water decreases. As a result, the upthrust acting due to water on hollow glass sphere also decreases, which causes it to sink.
(e) Inside the freezer, when the temperature of water falls below 4 °C , the water in the bottle starts expanding. If the bottle is completely filled and tightly closed, there is no space for water to expand, and hence, the bottle may burst.
MCQ-6(A) ICSE Physics Heat and Energy Solutions
Page 130
Question 1
Calorie is the unit of :
(a) Heat
(b) Work
(c) Temperature
(d) food
Answer 1
Calorie is the unit heat.
Question 2
1 J equals to:
(a) 0.24 cal
(b) 4.18 cal
(c) 1 cal
(d) 1 kcal
Answer 2
calorie = 4.186 J
Therefore,
1 J = 1/4.186 = 0.24 cal
Question 3
S.I. unit of temperature is:
(a) Cal
(b) Joule
(c) Celsius
(d) kelvin
Answer 3
The SI unit of temperature is kelvin (K).
Question 4
Water is cooled from 4 °C to 0 °C. It will:
(a) contract
(b) expand
(c) first contract, then expand
(d) first expand, then contract
Answer 4
Water shows anomalous behavior between 0 °C and 4 °C. Hence, when it is cooled it expands.
Question 5
Density of water is maximum at:
(a) 0°C
(b) 100°C
(c) 4°C
(d) 15°C
Answer 5
Water shows anomalous behavior between 0 °C and 4 °C. It has lowest volume at 4 °C. Hence, its density will be maximum at 4 °C.
Return to Concise Selina Physics ICSE Class-9 Solutions
Thanks
Please share with your friends


