Heat Transfer ICSE Class-8th Concise Selina Physics Solutions Chapter-6. We Provide Step by Step Answer of Objective, True False , Fill in the blanks,Match the following and Short/ Long Answer Question Type of Exercise-6 Heat Transfer. Visit official Website CISCE for detail information about ICSE Board Class-8.
Heat Transfer ICSE Class-8th Concise Selina Physics
-: Select Topic :-
A. Objective Questions Heat Transfer ICSE Class-8th Concise Selina
1. Write true or false for each statement
(a) Evaporation is rapid on a wet day.
Answer. False.
(b) Evaporation takes place only from the surface of liquid.
Answer. True.
Answer. False.
(d) Temperature of a liquid rises during boiling or vaporization
Answer. False.
(e) All molecules of a liquid take part in boiling.
Answer. True.
(f) Boiling is a rapid phenomenon.
Answer. True.
(g) All solids expand by the same amount when heated to the same rise in temperature.
Answer. False.
(h) Telephone wires are kept tight between the two poles in winter.
Answer. True.
(i) Equal volumes of different liquids expand by the different amount when they are heated to the same rise in temperature.
Answer. True.
j) Solids expand the least and gases expand the most on being heated.
Answer. True.
(k) A mercury thermometer makes use of the property of expansion of liquids on heating.
Answer. True.
(l) Kerosene contracts on heating.
Answer. False.
2. Fill in the blanks
(a) Boiling occurs at a fixed temperature.
(b) Evaporation takes place at all temperature.
(c) The molecules of liquid absorb heat from surroundings in evaporation.
(d) Heat is absorbed during boiling.
(e) Cooling is produced in evaporation.
(f) A longer rod expands more than a shorter rod on being heated to the same temperature.
(g) Liquids expand more than the solids.
(h) Gases expand more than the liquids.
(i) Alcohol expands more than water.
(j) Iron expands less than copper
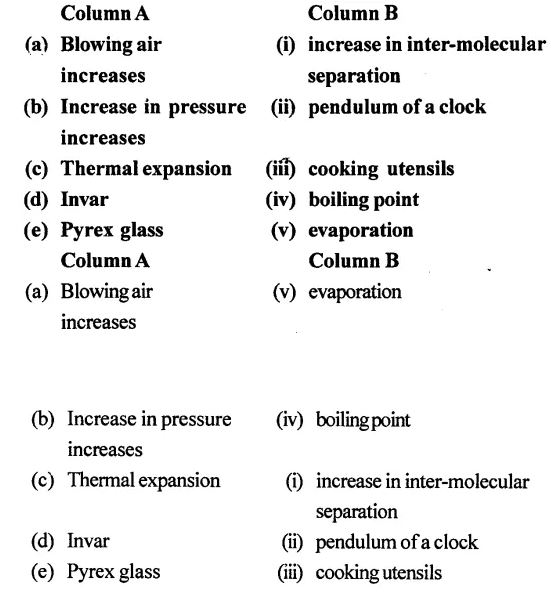
3. Match the following
4. Select the correct alternative
(a) In evaporation
- all molecules of liquid begin to escape out
- only the molecules at the surface escape out
- the temperature of liquid rises by absorbing heat from surroundings.
- the molecules get attracted within the liquid
Answer
only the molecules at the surface escape out
(b) The rate of evaporation of a liquid increases when :
- temperature of liquid falls
- liquid is poured in a vessel of less surface area
- air is blown above the surface of liquid
- humidity increases.
Answer
air is blown above the surface of liquid
c) During boiling or vaporization
- all molecules take part
- temperature rises
- no heat is absorbed
- the average kinetic energy of molecules increases.
Answer
increasing the pressure, on liquid
(d) The boiling point of a liquid is increased by
- increasing the volume of liquid
- increasing the pressure, on liquid
- adding ice to the liquid
- decreasing pressure on liquid.
Answer
increasing the pressure, on liquid
(e) Two rods A and B of the same metal, but of length 1 m and 2 m respectively, are heated from 0°C to 100°C. Then
- both the rods A and B elongate the same
- and the rod A elongates more than the rod B
- the rod B elongates more than the rod A
- the rod A elongates, but the rod B contracts.
Answer
the rod B elongates more than the rod A
(f) Two rods A and B of the same metal, same length, but one solid and the other hollow, are heated to the same rise in temperature.
Then
- the solid rod A expands more than the hollow rod B
- the hollow rod B expands more than the solid rod A
- the hollow rod B contracts, but the solid rod A expands
- both the rods A and B expand the same.
Answer
both the rods A and B expand the same.
(g) A given volume of alcohol and the same volume of water are heated from the room temperature to the same temperature then.
- alcohol contracts, but water expands
- water contracts, but alcohol expands
- water expands more than alcohol
- alcohol expands more than water.
Answer
alcohol expands more than water.
(h) The increase in length of a metal rod depends on
- the initial length of the rod only
- the rise in temperature only
- the material of rod only
- all the above three factors.
Answer
all the above three factors
(i) The correct statement is
- Iron rims are cooled before they are placed on the cart wheels.
- A glass stopper gets tighten on warming the neck of the bottle.
- Telephone wires sag in winter, but become tight in summer.
- A little space is left between two rails on a railway track.
Answer
A little space is left between two rails on a railway track
Short/ Long Answer Question Chapter-6 Heat Transfer Selina Physics for class-8
Page-120
Question-1 :-
What is matter? What is it composed of.
Answer-1 :-
Anything that has mass, occupies volume and can be felt by our senses is matter. It is composed of molecules.
Question-2 :-
Name the three states of matter and distinguish them on the basis of their (i) volume, and (ii) shape
THREE STATES OF MATTER:
(i) SOLID
(ii) LIQUID
(iii) GASEOUS
DISTINCTION BETWEEN THREE STATES ON THE BASES OF
(i) VOLUME:
SOLID: have a definite volume.
LIQUID: have a definite volume.
GASEOUS: have no definite volume.
(ii) SHAPE:
SOLID: Have a definite shape.
LIQUID: Have no definite shape.
GASEOUS: Have no definite shape
Question-3 :-
Distinguish between liquid and vapour (or gas) states of matter on the basis of the following factors
(a) Arrangement of molecules
(b) Inter-molecular separation
(c) Inter-molecular force, and
(d) Kinetic energy of molecules
Answer-3 :-
DISTINCTION BETWEEN LIQUID AND VAPOUR ON THE BASES OF:
| LIQUID | VAPOUR | |
| (a) Arrangement of molecules | Closely packed | Very loosely packed |
| (b) Intermolecular separation | Least | Maximum |
| (c) Intermolecular force | Maximum | Least |
| (d) Kinetic energy of molecules | Least | Maximum |
Question-4 :-
What is evaporation? Explain it on the basis of molecular motion.
Answer-4 :-
The change of liquid into its vapor at all temperatures from its surface is called evaporation.
The intermolecular spaces in liquids are more and the molecular force of attraction is less which makes them move throughout the liquid.
They cannot escape the surface of liquids because of less kinetic energy, when heated they acquire sufficient kinetic energy and they overcome the attractive forces of other molecules. On escaping the molecules form the vapor of the liquid.
Question-5 :-
Do all molecules of a liquid take part in evaporation? If not, explain your answer.
Answer-5 :-
No, not all molecules of liquid do not take part in evaporation, those molecules which acquire sufficient kinetic energy escape the surface by overcoming forces of attraction of other molecules.This continues till all the liquid evaporates.
Question-6 :-
No heat is supplied to a liquid during evaporation. How does then the liquid change into its vapours?
Answer-6 :-
When the molecules liquid collide with each other they acquire kinetic energy and they overcome the attractive forces of other molecules and change into vapours. The particles of water on the surface absorb heat from the surroundings and change into vapour.
Question-7 :-
Comment on the statement, ‘evaporation is a surface phenomenon’.
Answer-7 :-
Evaporation is the change of liquid into vapours at all temperatures from the surface. It takes place at surface as molecules which are at surface and gain sufficient kinetic energy from the surroundings above to overcome attractive forces.
Question-8 :-
Why is cooling produced when a liquid evaporates?
Answer-8 :-
To change liquid into vapours, heat is needed which is taken from surroundings and temperature of the container or body itself falls and cooling is produced.
Short/ Long Answer Question Chapter-6 Heat Transfer Selina Physics for class-8
Page-121
Question-9 :-
Give reasons for the increase in rate of evaporation of a liquid
(a) When air is blown above the liquid.
(b) When surface area of liquid is increased
(c) When temperature of liquid is increased.
Answer-9 :-
(a) The factors that decide the rate of evaporation are:
- Temperature
- Surface area exposed
- Partial pressure of liquid in the air above it.
When air is blown above the surface of liquid, it will take away the liquid carrying air particles from the air above the liquid, resulting in decrease in humidity and increase in rate of evaporation.
(b) The factors that decide the rate of evaporation are:
- Temperature
- Surface area exposed
- Partial pressure of liquid in the air above it.
On increasing the surface area, the number of molecules in contact at the surface of liquid increases, and evaporation takes place rapidly.
(c) The factors that decide the rate of evaporation are:
- Temperature
- Surface area exposed
- Partial pressure of liquid in the air above it.
The increase in temperature increases the kinetic energy of the molecules, they escape the force of attraction of molecules and evaporate faster.
Question-10 :-
What is boiling? Explain it on the basis of molecular motion?
Answer-10 :-
The change of liquid to vapours on heating at a constant temperature is called boiling.
The kinetic energy of molecules determines the molecular motion. On heating, the kinetic energy of molecules of liquid increases.These molecules start moving more rapidly and away from each other. Thus, converting from liquid to gas.
Question-11 :-
Why does bubbles appear when a liquid is heated?
Answer-11 :-
On heating, liquid starts changing into its vapour state at the place where it is being heated. The liquid converts to gas which appears in the form of bubbles.
Question-12 :-
What is the change in average kinetic energy of molecules of a liquid during boiling at its boiling point?
Answer-12 :-
The kinetic energy is the measure of temperature of the body, when temperature increases the average kinetic energy also increases. At boiling point, average kinetic energy increases.
Question-13 :-
How is the heat energy supplied to a liquid used during boiling at a fixed temperature?
Answer-13 :-
During boiling, the heat energy supplied is used in increasing the energy of the water molecule, which changes its state from liquid to gaseous.
Question-14 :-
Name two ways of change of liquid state to the vapour state and distinguish them.
Answer-14 :-
Two ways of change of liquid state to the vapour state are:
(i) Boiling
(ii) Evaporation
| Boiling | Evaporation |
|---|---|
| Heat is supplied | Heat is absorbed from the surroundings |
| Fast process | Slow process |
| Starts from bottom | Starts from surface |
| Sound is produced | Silent process |
| Heating effect | Cooling effect |
| At a fixed temperature | At all temperatures |
Question-15 :-
What do you understand by thermal expansion of a substance?
Answer-15 :-
The expansion of a substance on heating is called thermal expansion of the substance.
Question-16 :-
Give two examples of the substance which expand on heating.
Answer-16 :-
Iron and water expand on heating.
Question-17 :-
Describe an experiment to demonstrate the thermal expansion in solids.
Answer-17 :-
To show – Thermal expansion in solids
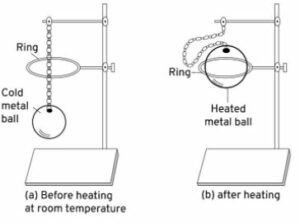
Experiment – Gravesand’s ball and ring experiment
Take a metallic ball and ring such that the ball can pass through the ring easily. The ball is heated, and the ball cannot pass through the ring.
This is because the solid ball expands in size. On cooling, it can pass through the ring.
Observation – Solids expand on heating and contract on cooling.
Question-18 :-
State three factors on which depend the linear expansion of a metal rod on heating.
Answer-18 :-
Factors on which linear expansion of rod depends:
(i) Length of rod
(ii) Temperature of rod
(iii) Nature of material of rod
Question-19 :-
Two iron rods – one 10 m long and other 5 m long, are heated to the same rise in temperature. Which will expand more?
Answer-19 :-
Lt – L0 ∝ L0
∴ The rod having greater length will expand more when rods are heated to the same temperature.
∴ Rod with 10 m length will expand more.
Question-20 :-
Two identical rods of copper are heated to different temperatures – one by 5°C and the other by 10°C. Which rod will expand more?
Answer-20 :-
Two rods with the same length and material are heated to different temperatures.
Rod with higher temperature i.e 10°C will expand more.
Question-21 :-
One rod of copper and another identical rod of iron are heated to the same rise in temperature. Which rod will expand more? Give reason.
Answer-21 :-
Expansion depends on the nature of material, when two identical rods are of different material and heated to the same rise in temperature, the copper rod will expand more than the iron rod as copper is a better conductor of heat than iron.
Question-22 :-
Two identical rods – one hollow and the other solid, are heated to the same rise in temperature. Which will expand more?
Answer-22 :-
When two identical rods are heated at the same temperature, they will expand by the same amount on heating as the material of the rods are the same.
Question-23 :-
In the ball and ring experiment, if the ball after heating is left to cool on the ring for sometime, the ball again passes through the ring. Explain the reason.
Answer-23 :-
On heating, the solid ball expands and increases in size and cannot pass through the ring. The ball after heating is left to cool on the ring for sometime, the ball again passes through the ring, because it contracts in size and passes through the ring.
Question-24 :-
Explain the following:
(a) The telephone wires break in winter.
(b) Iron rims are heated before they are fixed on the wooden wheels.
(c) The gaps are left between the successive rails on a railway track.
(d) A glass stopper stuck in the neck of a bottle be removed by pouring hot water on the neck of the bottle.
(e) A cement floor is laid in small pieces with gaps in between.
Answer-24 :-
(a) Most telephone wires are made out of copper, which contracts with drop in temperature and hence breaks in winter if it becomes too tight between the poles.
(b) Iron rims are made smaller in diameter than the wooden wheel, so on heating the rims expand and can easily slip over the wooden wheel and contract on cooling and fit tight over the wooden wheel.
(c) The gaps are left between the successive rails for making space for their expansion when there is rise in temperature in summers.
(d) A glass stopper stuck in the neck of a bottle can be removed by pouring hot water on the neck of the bottle, because the neck expands on pouring hot water.
(e) Small pieces of cement expand in summers. So, to allow expansion, gaps are left between small pieces.
Question-25 :-
Why is one end of a steel girder in a bridge kept on rollers instead of fixing it in pillar?
Answer-25 :-
When temperature increases, the metal bridge expands and the rollers slide to allow for expansion otherwise the bridge may break the pillar.
Question-26 :-
A metal plate is heated. State three factors on which increase in its area will depend.
Answer-26 :-
Factors on which increase of metal plate area depends are:
(i) Original area
(ii) Rise in temperature
(iii) Nature of material of plate.
Question-27 :-
A cubical metal solid block is heated. How will its volume change?
Answer-27 :-
When a solid is heated, it expands in all directions. When a cubical metal solid block is heated the volume of the cube also increases.
Question-28 :-
Describe an experiment to show that liquids expand on heating.
Answer-28 :-
To show – Liquids expand on heating
Experiment – Take a round bottom flask and fill it with water. Close its mouth with a cork having a delivery tube and mark the level of water as A.
Now heat the flask, we see that the level of water in the tube rises from A to a higher mark B.
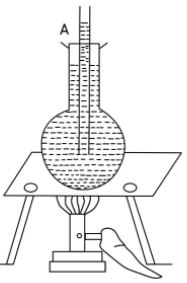
Observation – This shows that liquids expand on heating.
Question-29 :-
State one application of thermal expansion of liquids.
Answer-29 :-
A mercury thermometer is based on the application of thermal expansion of liquids. The bulb of the thermometer is kept in contact with a hot body, and mercury expands and level of mercury rises in the tube.
Question-30 :-
Describe an experiment to show that air expands on heating.
Answer-30 :-
To show – Air expands on heating
Procedure – Take a glass flask with flat bottom and close its mouth with a cork having a capillary tube containing indicator visible in the tube. Keep it air tight. Now place the flask in hot water. This happens because air inside the flask expands with rise in temperature.
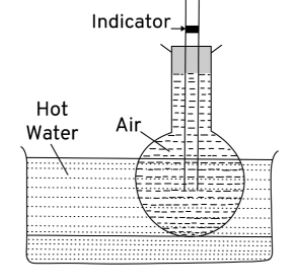
Observation – This shows that air expands on heating.
Question-31 :-
An empty glass bottle is fitted with a narrow tube at its mouth. The open end of the tube is kept in a beaker containing water. When the bottle is heated, bubbles of air are seen escaping into water. Explain the reason.
Answer-31 :-
On heating, the air in the bottle expands and escapes the water in the form of bubbles.
Question-32 :-
Which of the following will expand more, when heated to the same temperature: (a) solid (b) liquid and (c) gas?
Answer-32 :-
Gas will expand more as the intermolecular forces of attraction in gas molecules is less and kinetic energy is maximum.
Question-33 :-
Describe an experiment to show that the same volume of different liquids heated to the same rise in temperature expand by different amounts.
Answer-33 :-
To show – Cubical expansion of different liquids is different

Experiment – Take four identical glass flasks each fitted with a narrow glass tube through a cork at its mouth. Fill flask A with water, B with kerosene, C with alcohol, D with Benzene. Keep all 4 flasks in a hot water bath with enough hot water in it. After some time we will see that different liquids rise to different levels. Water expands the least and benzene expands the most.
Observation – Different liquids heated to the same rise in temperature expand by different amounts.
Question-34 :-
100 ml of each of the following liquid is heated from 10°C to 50°C. Which will expand more (a) water (b) benzene (c) alcohol?
Answer-34 :-
Benzene will expand the most.
Question-35 :-
Water is heated from 0°C to 4°C. Will it expand?
Answer-35 :-
Though liquids expand on heating, water does not expand between 0°C to 4°C instead it contracts as water has maximum density at 4°C. It expands above 4°C.
Question-36 :-
What do you mean by anomalous behaviour of water?
Answer-36 :-
Substances expand on heating, and their density decreases. Water does not expand between 0°C to 4°C instead it contracts. It expands above 4°C. This means water has maximum density at 4°C. This is called anomalous behaviour of water.
Question-37 :-
How does the density of a substance (solid, liquid and gas ) change on heating?
Answer-37 :-
Density = Mass/Volume
When a substance is heated its volume increases and so the density decreases. In solids, increase in volume is negligible and hence decrease in density too. In liquids and gases, as the temperature increases, volume increases and therefore density decreases considerably.
Question-38 :-
An iron washer is heated.
(a) State the effect on its mass.
(b) State the effect on its internal diameter
(c) State the effect on its external diameter
(d) State the effect on its density.
Answer-38 :-
(a) Mass does not change in heating.
(b) Internal diameter increases as the washer expands.
(c) External diameter increases as the washer expands.
(d) Density decreases as volume increases.
Return to- ICSE Class -8 Selina Concise Physics Solutions
Thanks
Please share with your friends .

Nice link
thanks a lot
Good site for reference
thanks a lot