Hydrogen Chloride ICSE Class-10 Concise Chemistry Selina Solutions Chapter-8 Study of Compound-A. We Provide Step by Step Answer of Exe-8 and Previous Year Questions of Exercise-8 Hydrogen Chloride Study of Compound-A ICSE Class-10 . Visit official Website CISCE for detail information about ICSE Board Class-10.
| Board | ICSE |
| Publications | Selina Publishers PVT LTD |
| Subject | Concise Chemistry |
| Class | 10th |
| writer | Dr SP Singh |
| Chapter-8 | Study of Compound A Hydrogen Chloride |
| Topics | Solutions of Exercise-8 Questions |
| Edition | 2021-2022 |
Hydrogen Chloride ICSE Class-10 Concise Chemistry Selina Solutions Chapter-8
Study of Compound-A
-: Select Topics :-
How to solve Hydrogen Chloride in Chemistry for ICSE Class 10
Read the Chapter -8 Hydrogen Chloride Study of Compound-A carefully Focus on reaction involve in Chemical Properties of HCL. Various Step involve in Purification of HCL Gas. Uses of Inverted Funnel in HCL. Lab Preparation of HCL Gas. Use of Fountain Experiment .
Hydrogen Chloride ICSE Class-10 Exercise- 8
Page-147
Question -1
Draw a labelled diagram for the laboratory preparation of hydrogen chloride gas and answer the following
(a) Name the acid used. Why is this particular acid preferred to other acids? (2018)
(b) Give the balanced equation for the reaction.
(c) Name the drying agent used in drying hydrogen chloride gas.
(d) Phosphorous penta oxide and calcium oxide are good drying agents, but they cannot be used to dry hydrogen chloride gas. Why?
(e) Why is direct absorption of HCl gas in water not feasible?
(f) What arrangement is done to dissolve HCl gas in water?
Answer 1
(a) Concentrated H2SO4
(b) The balanced equation for the reaction:
(c) The drying agent used in drying hydrogen chloride gas is conc. sulphuric acid.
(d) Phosphorous pentoxide and calcium oxide are good drying agents, but they cannot be used to dry hydrogen chloride gas because they react with hydrogen chloride.
![]()
(e) Hydrogen chloride gas is highly soluble in water. Therefore, it is not collected over water.
(f) The funnel arrangement is done to dissolve HCl gas in water.
Question 2
Explain why:
(a) Anhydrous HCl is a poor conductor while aq. HCl is an excellent conductor.
(b) When the stopper of a bottle full of hydrogen chloride gas is opened there are fumes in the air.
(c) A solution of hydrogen chloride in water turns blue litmus red and conducts electricity , while a solution of the same gas in toluene:
(i) Has no effect on litmus ,and
(ii) Does not conduct electricity
(d) Thick white fumes are formed when glass rod dipped in NH4OH is brought near the mouth of bottle full of HCl gas.
(e) Dry hydrogen chloride gas does not affect a dry strip of blue litmus paper but it turns red in the presence of drop of water.
(f) Hydrogen chloride gas is not collected over water.
Answer 2
(a) Anhydrous HCl is poor conductor due to the absence of ions in it whereas aqueous HCl is excellent conductor since it contains ions.
(b) When the stopper is opened HCl gas comes in contact with water vapors of air and gives white fumes due to the formation of hydrochloric acid.
(c) A solution of HCl in water gives hydronium ions and conducts electricity, but HCl is also soluble in dry toluene, but in that case it neither (i) turns blue litmus red (ii) nor does conducts electricity. This indicates the absence of H+ ions in toluene showing thereby that hydrogen chloride is a covalent compound.
(d) When ammonium hydroxide is brought near the mouth of HCl, dense white fumes are formed due to the formation of ammonium chloride.
HCl + NH4OH —–→ NH4Cl + H2O
(e) Dry hydrogen chloride is not acidic whereas moist Hydrogen chloride is acidic. In presence of a drop of water HCl gas dissolves in water and forms hydrochloric acid which turns blue litmus paper red.
(f) Hydrogen chloride is not collected over water as it is highly soluble in water.
Page-148
Question 3
The given set up of the figure is for preparation of an acid.
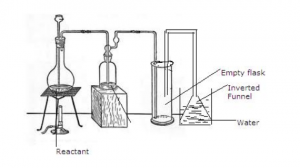
(a) Name the acid prepared by this method.
(b) Name the reactants used.
(c) Why empty flask is used.
(d) What is drying agent used? Why is this drying agent chosen?
(e) What is the role of inverted funnel in the arrangement?
Answer 3
(a) Hydro chloric acid is prepared by this method.
(b) The reactants are sodium chloride and Sulphuric acid.
(c) The empty flask acts as Anti-Suction device. In case the back suction occurs the water will collect in it and will not reach the generating flask.
(d) The drying agent is Conc. Sulphuric acid. Sulphuric acid is chosen as drying agent because it does not react with HCl.
(e) The Inverted funnel :
Prevents or minimizes back suction of water.
Provides a large surface area for absorption of HCl gas.
Question 4
(a)
(i) Name the experiment illustrated below.
(ii) State the colour of the water that has entered the round-bottomed flask.
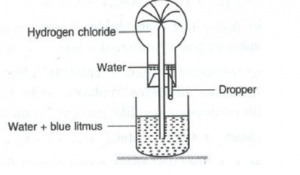
(b) What property of hydrogen chloride is demonstrated when it is collected by downward delivery (Upward displacement)?
Answer 4
(a)
(i) The experiment is the fountain experiment and is used to demonstrate solubility.
(ii) The colour of the water that has entered the round-bottomed flask is red.
(b) When hydrogen chloride is collected by downward delivery or upward displacement, it shows that it is heavier than air.
Question 5
(a) Name an element which reacts with hydrogen to form a compound which is strongly acidic in water.
(b) Explain why dilute hydrochloric acid cannot be concentrated by boiling beyond 22.2%.
Answer 5
(a) Hydrogen reacts with chlorine to form hydrogen chloride.
(b) Dilute hydrochloric acid cannot be concentrated by boiling beyond 22.2% because molecules of HCl(g) get mixed with water vapour.
Question 6
How will you prove that Hydrochloric acid contains (i) Hydrogen (ii) Chlorine.
Write equations for the reactions.
Answer 6
We can prove that hydrochloric acid contains both hydrogen and chlorine by the following experiment.
Take a voltameter used for electrolysis of water, fitted with platinum cathode and graphite anode.
Into the voltameter pour 4 molar HCl and pass direct current.
It is seen that a colourless gas is evolved at cathode and a greenish gas is evolved at anode.
When a burning splinter is brought near a colourless gas, it bursts into flame thereby proving that it is hydrogen gas.
When moist starch iodide paper is held in the greenish yellow gas, it turns blue black, thereby proving that the gas is chlorine.
2HCl ? H2 + Cl2
This experiment proves that hydrochloric acid contains both hydrogen and chlorine.
Question 7
Name
(a) A Black metallic oxide which reacts with hydrochloric acid to give a coloured solution.
(b) Two colourless gases, which when mixed produce a white solid.
(c) Two gases which chemically combine to form liquid.
(d) A chloride which is soluble in excess of ammonium hydroxide.
(e) The chemical in which gold can be dissolved.
(f) the experiment which demonstrates that hydrogen chloride is soluble in water.
(g) the gas produced when chlorine water is exposed to sunlight.
Answer 7
(a) Manganese dioxide
(b) Hydrogen chloride and ammonia
(c) Hydrogen and oxygen
(d) AgCl(Silver chloride)
(e) Aqua regia
(f) Fountain experiment
(g) Hydrogen chloride gas
Question 8
Solution A reacts with an acid B (which gives greenish yellow gas on reacting with oxidizing agents like Pb3O4) to give white precipitate C insoluble in nitric acid but soluble in ammonium hydroxide. Name A, B and C.
Answer 8
A is Silver nitrate
B is Hydrochloric acid
C is Silver chloride
Question 9
Complete and balance the following reactions, state whether dilute or conc. acid is used.
(a) NH4OH + HCl![]()
(b) NaHSO3 + HCl![]()
(c) Pb(NO3)2 +HCl![]()
(d) Pb3O4 + HCl![]()
Answer 9
(a) NH4OH + HCl ![]() NH4Cl + H2O
NH4Cl + H2O
(b) NaHSO3 + HCl ![]() NaCl + H2O + SO2
NaCl + H2O + SO2
(c) Pb(NO3)2 +2HCl ![]() PbCl2 +2HNO3
PbCl2 +2HNO3
(d) Pb3O4 + 8HCl ![]() 3PbCl2 +4H2O +Cl2
3PbCl2 +4H2O +Cl2
Question 10
How will the action of dilute hydrochloric acid enable you to distinguish between the following:
(a) Sodium carbonate and sodium sulphite.
(b) Sodium thiosulphate and sodium sulphite.
Answer 10
(a) Sodium carbonate on treating with dil.HCl results in the formation of sodium chloride with the liberation of carbon dioxide gas.
Na2CO3 + 2HCl → 2NaCl + H2O + CO2 ↑
Sodium sulphite on treating with dil.HCl results in the formation of sodium chloride with the liberation of sulphur dioxide gas.
Na2SO3 + 2HCl → 2NaCl + H2O + SO2 ↑
(b) Sodium thiosulphate reacts with dil. HCl to produce sulphur dioxide gas and precipitates yellow sulphur.
Na2S2O3 + 2HCl → 2NaCl + H2O + SO2 + S↓
Sulphur is not precipitated when sulphites are treated with dil. HCl.
Question 11
Give three distinct tests (apart from using an indicator) you would carry out with solution of HCl to illustrate the typical properties of an acid.
Answer 11
Three tests are:
HCl gas gives thick white fumes of ammonium chloride when glass rod dipped in ammonia solution is held near the vapours of the acid.
NH3 + HCl ![]() NH4Cl
NH4Cl
With silver nitrate HCl gives white precipitate of silver chloride. The precipitate is insoluble in nitric acid but soluble in ammonium hydroxide.
AgNO3 + HCl ![]() AgCl + HNO3
AgCl + HNO3
A greenish yellow gas is liberated when concentrated hydrochloric acid is heated with oxidizing agent like manganese dioxide.
MnO2 + 4HCl MnCl2 +2H2O + Cl2
Question 12
MnO2, PbO2 and red lead react with conc. HCl acid liberates Cl2.
What is the common property being shown by these metal oxides?
Answer 12
MnO2, PbO2 and red lead react with conc. HCl acid to liberate Cl2. This shows that hydrochloric acid is oxidized to chlorine by oxidizing agents.
Question 13
State which of the two – a solution of HCl in water or in toluene -is an electrolyte. Explain.
Answer 13
When hydrogen chloride gas is dissolved in water, hydrochloric acid is formed. The covalent compound ionises in water because of its polar nature and it can conduct electricity.
![]()
Hydrogen chloride gas is soluble in toluene, but there is an absence of H3O+ in toluene, so it does not ionise the gas; thus, it cannot conduct electricity.
Question 14
Convert
(a) two soluble metallic nitrates to insoluble metallic chlorides using dil. HCl
(b) Hydrochloric acid to nascent chlorine
Answer 14
(a )Conversion of metallic nitrates to insoluble metallic chlorides using dil. HCl:
(i) ![]()
(ii) ![]()
(b) Hydrochloric acid to nascent chlorine
Hydrochloric acid can be converted to nascent chlorine by mixing three parts of concentrated hydrochloric acid and one part of concentrated nitric acid.
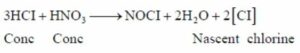
Question 15
A solution of hydrogen chloride in water is prepared. The following substances are added to separate portions of the solution:
| S.No. | Substances
added |
Gas
evolved |
Odour |
| 1.
2. 3. 4. |
Calcium carbonate
Magnesium ribbon Manganese(IV) oxide with heating Sodium sulphide |
_______ | ______ |
Complete the table by writing the gas evolved in each case and its odour.
Answer 15
| S.No. | Substances added | Gas evolved | Odour |
| 1.
2. 3. 4.
|
Calcium carbonate
Magnesium ribbon Manganese(IV) oxide with heating Sodium sulphide |
Carbon dioxide
Hydrogen Cl2 Hydrogen sulphide |
Odourless
Odourless Strong Pungent odour Rotten egg |
page-149
Question 16
State the composition of aqua regia. State which component is the oxidizing agent in aqua regia.
Answer 16
A mixture having three parts of conc. Hydrochloric acid and one part of conc. Nitric acid is called aqua-regia.
Nitric acid acts as oxidizing agent.
Question 17
Write an equation for the reactions of hydrochloric acid on
(a) silver nitrate solution
(b) magnesium foil
(c) caustic soda solution
(d) zinc carbonate
(e) manganese (IV) oxide
(f) copper oxide
Answer 17
Equations for the reactions of hydrochloric acid on
(a) silver nitrate
![]() solution
solution
(b) magnesium foil
![]()
(c) caustic soda solution
![]()
(d) zinc carbonate
![]()
(e) manganese (IV) oxide
![]()
(f) copper oxide
![]()
Question 18
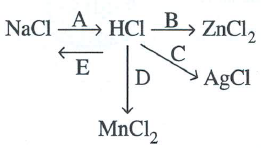
Study the flow chart and give balanced equations with conditions for the conversions A, B, C,D and E.
Answer 18
NaCl + H2S![]() O4 NaHSO4 + HCl
O4 NaHSO4 + HCl
Fe + 2HCl ![]() FeCl2 + H2
FeCl2 + H2
HCl + NH3 ![]() NH4Cl
NH4Cl
PbO2 + 4HCl ![]() PbCl2 + 2H2O + Cl2
PbCl2 + 2H2O + Cl2
Question 19
Write the balanced equations for the reaction of dilute hydrochloric acid with each of the following:
(a) Iron
(b) Sodium hydrogen carbonate
(c) Iron(II) sulphide
(d) magnesium sulphite (2018)
Answer 19
(a) Fe +2HCl ![]() FeCl2 +H2
FeCl2 +H2
(b) NaHCO3 + HCl ![]() NaCl + H2O + CO2
NaCl + H2O + CO2
(c) FeS + 2HCl ![]() FeCl2 + H2S
FeCl2 + H2S
(d) 
Question 20
Write observation
Lead nitrate solution is mixed with dilute hydrochloric acid and heated.
Answer 20
(a) Lead nitrate solution is mixed with dilute hydrochloric acid and heated (2018)
(b) Small piece of zinc is added to dil hydrochloric acid
Question-21
(a) drying agent is used to dry HCl gas is
A………….
B…………..
C…………..
D…………….
(b) when sodium chloride is heated with concentrated sulphuric acid below 200 C one of the product form is (sodium hydrogen sulphate /sodium sulphate / chlorine) (2019)
Answer-21
(a) drying agent is used to dry HCl gas is….option A. Concentrated H2SO4……..
(b) when sodium chloride is heated with concentrated sulphuric acid below 200 C one of the product form is ….sodium hydrogen sulphate……… (2019)
Previous Year Question of Exercise -8, Hydrogen Chloride Selina Solutions for ICSE Class-10 Chemistry
Question 2010
(a) Aqua regia is a mixture of
(i) Dilute hydrochloric acid and concentrated nitric acid
(ii) Concentrated hydrochloric acid and dilute nitric acid
(iii). Concentrated hydrochloric acid [1 part] and concentrated nitric acid [3 parts]
(iv) Concentrated hydrochloric acid [3 parts] and concentrated nitric acid [1 part]
(b) How would you distinguish between diluteHCl and dilute HNO3 by addition of only one solution?
(c) Name two gases which can be used in the study of the fountain experiment. State the common property demonstrated by the fountain experiment.
Answer 2010
(a) Aquaregia is a mixture of Concentrated hydrochloric acid [3 parts] and concentrated nitric acid [1 part].
(b) Silver nitrate solution will give a white ppt. when added to dil. hydrochloric acid and no change will be observed when added to dil. nitric acid.
(c) Hydrogen chloride and ammonia gas.
Question 2011
(a) Choose the correct answer from the choices given: Hydrogen chloride gas being highly soluble in water is dried by
(i) Anhydrous calcium chloride
(ii) Phosphorous pentoxide
(iii) Quicklime
(iv) Conc. sulphuric acid
(b) Write the balanced chemical equation.
(i) Sodium thiosulphate is reacted with dilute hydrochloric acid.
(ii) Calcium bicarbonate reacts with dilute hydrochloric acid.
(c) In the laboratory preparation of hydrochloric acid, hydrogen chloride gas is dissolved in water.
(i) Draw a diagram to show the arrangement used for the absorption of HCl gas in water.
(ii) State why such an arrangement is necessary. Give two reasons for the same.
(iii)Write balanced chemical equations for the laboratory preparation of HCl gas when the reaction is A. Below 200°C; B. Above 200°C.
Answer 2011
(a) Hydrogen chloride gas being highly soluble in water is dried by conc. sulphuric acid.
(b) Balanced chemical equations:
(i) Sodium thiosulphate is reacted with dilute hydrochloric acid:
![]()
(ii) Calcium bicarbonate reacts with dilute hydrochloric acid:
![]()
(c)
(i) Diagram to show the arrangement used for the absorption of HCl gas in water:
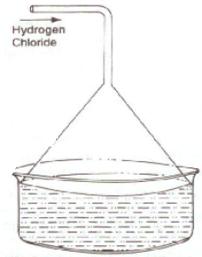
(ii) Such an arrangement is necessary to prevent back suction of water into the apparatus and it provides a large surface area for dissolution of hydrogen chloride gas.
(iii) Balanced chemical equations for the laboratory preparation of HCl gas:
![]()
![]()
Question 2012
(a) Rewrite the correct statement with the missing word/s:Aqua regia contains one part by volume of nitric acid and three parts by volume of hydrochloric acid.
(b) Give reason for the following: Hydrogen chloride gas cannot be dried over quicklime.
(c) Give a balanced equation for the reaction: Conc. hydrochloric acid and potassium permanganate solution.
(d) Give balanced equations with conditions, if any, for the following conversions:
(i) Sodium chloride → Hydrogen chloride
(ii) Hydrogen chloride → Iron (II) chloride
(iii)Hydrogen chloride → Ammonium chloride
(iv) Hydrogen chloride → Lead chloride
Answer 2012
(a) Aquaregia contains one part by volume of conc. nitric acid and three parts by volume of conc. hydrochloric acid.
(b) Because HCl undergoes a chemical reaction with quicklime.
2HCl + CaO → CaCl2 + H2O
(c) Balanced equation for the reaction of conc. hydrochloric acid and potassium permanganate solution:
![]()
(d) Balanced equations with conditions for the following conversions:
(i) Sodium chloride→ Hydrogen chloride
![]()
(ii) Hydrogen chloride→ Iron (II) chloride
![]()
(iii) Hydrogen chloride → Ammonium chloride
![]()
(iv) Hydrogen chloride→ Lead chloride
![]()
Question 2013
(a) Identify the gas evolved when
(i) Potassium sulphite is treated with dilute hydrochloric acid.
page-149
(ii) Concentrated hydrochloric acid is made to react with manganese dioxide.
(b) State one appropriate observation for
(i) Copper sulphide is treated with dilute hydrochloric acid.
(ii) A few drops of dil. HCl are added to AgNO3 solution, followed by addition of NH4OH solution.
Answer 2013 ( Hydrogen Chloride ICSE Class-10 )
(a) i. When potassium sulphite is treated with dilute hydrochloric acid, sulphur dioxide gas is evolved.
![]()
(ii) When concentrated hydrochloric acid is made to react with manganese dioxide, chlorine gas is evolved.
![]()
(b)
(i).Hydrogen sulphide gas is evolved which has the smell of rotten eggs.
(ii) A white precipitate of silver chloride is formed which is soluble in ammonium hydroxide.
Question 2014
(a) Fill in the blank from the choices in the brackets:
Quicklime is not used to dry HCl gas because [CaO is alkaline, CaO is acidic, CaO is neutral].
(b) Write the balanced equation for
Action of dilute hydrochloric acid on sodium sulphide.
(c) State your observation:
Dilute HCl is added to sodium carbonate crystals.
(d) Study the given figure and answer the questions that follow:
(i) Identify the gas Y.
(ii) What property of gas Y does this experiment demonstrate?
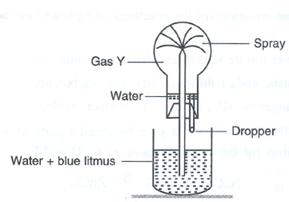
(iii). Name another gas which has the same property and can be demonstrated through this experiment.
Answer 2014
(a) Quicklime is not used to dry HCl gas because CaO is alkaline.
(b) Action of dilute hydrochloric acid on sodium sulphide:
![]()
(c) Dilute HCl is added to sodium carbonate crystals:
Sodium carbonate crystals on reaction with dilute HCl form sodium chloride, water and carbon dioxide gas, which is evolved with brisk effervescence. This is a neutralisation reaction because sodium carbonate is a basic salt, while hydrochloric acid is an acid. The chemical equation for this reaction is as follows:
![]()
(d)
(i) The gas is HCl (hydrogen chloride) gas.
(ii) The extreme solubility of hydrogen chloride gas is demonstrated by the fountain experiment.
(iii). Another gas which has the same property and can be demonstrated through this experiment is ammonia gas.
Question 2015
(a) Name the acid which on mixing with silver nitrate solution produces a white precipitate which is soluble in excess of ammonium hydroxide.
(b) Name the gas which produces dense white fumes with ammonia gas.
(c) The following questions pertain to the laboratory preparation of hydrogen chloride gas.
(i) Write the equation for its preparation, mentioning the conditions required.
(ii) Name the drying agent used in the above preparation and give a reason for the choice.
(iii) State a safety precaution taken during the preparation of hydrochloric acid
Answer 2015
(a) Hydro chloric acid (HCl)
(b) Hydrogen chloride
(c)
(i)![]()
(ii) For purification of HCl, it is drie on passing through conc. Sulphuric acid. It is preferre over the other drying agent because it does not react with HCl
(iii) i. Temperature maintaine at nearly 200oC.
ii Delivery tube dipp in drying agent i.e., conc. H2SO4.
iii. The lower end of the thistle funnel must be dipped in conc. Sulphuric acid.
Question 2016
(a) The aim of the Fountain experiment is to prove that :
(i) HCl turns blue litmus red
(ii) HCl is denser than air
(iii) HCl is highly soluble in water
(iv) HCl fumes in moist air
(b) Write a balanced chemical equation:
Action of hydrochloric acid on sodium bicarbonate.
(c) State your observations when :
(i) Dilute Hydrochloric acid is added to Lead nitrate solution and the mixture is heated.
(ii) Dilute Hydrochloric acid is added to Sodium thiosulphate
(d)Identify the gas evolved and give the chemical test in each of the following cases :
(i) Dilute hydrochloric acid reacts with iron (II) sulphide.
(ii) Dilute hydrochloric acid reacts with sodium sulphite.
Answer 2016
(a) Option C
HCl is highly soluble in water.
HCl is highly soluble in water. fountain experiment prove
(b) NaHCO3(s) + HCl(l)→ NaCl(aq) + H2O(l) + CO2(g)
(c)
(i) Hydrogen sulphide
The gas released has a rotten egg smell.
(ii) Sulphur dioxide
Freshly prepared K2Cr2O7 paper changes from orange to green.
— end of Hydrogen Chloride selina Concise ICSE Chemistry Class-10 :–
Return to :– Concise Selina Solutions for ICSE Chemistry Class-10
Thanks
Please Share with Your Friends