ICSE Chemistry Sem-2 Answer Key 2022, Solved Board Question Paper, Step by step solutions of ICSE Class-10 Chemistry Question Paper of Sem-2 for 2022 as council prescribe guideline. Visit official website CISCE for detail information about ICSE Class-10
ICSE Chemistry Sem-2 Answer Key 2022, Solved Board Question Paper,
| Board | ICSE |
| Class | 10th (X) |
| Subject | Chemistry |
| Topic | Solutions of Board Question Paper |
| Syllabus | on bifurcated syllabus (after reduction) |
| session | 2021-22 |
| Question Type | MCQ (Sec- A) and Descriptive (Sec-B) |
| Exam | Sem-2 |
| Max mark | 40 |
Section A
(Attempt all questions from this section)
Question 1:
Choose the correct answers to the questions from the given options. (Do not copy the question. Write the correct answer only.)
(i) The ore of Aluminium is:
(a) Calamine
(b) Haematite
(c) Magnetite
(d) Cryolite
Answer : (d) Cryolite
(ii) Hydrogen chloride gas is not collected over water, as:
(a) It is highly soluble in water.
(b) It is less soluble in water
(c) It is lighter than air.
(d) It is heavier than air.
Answer : (a) It is highly soluble in water.
(iii) An aqueous solution of ammonia is
(a) Neutral
(b) Acidic
(c) Basic
(d) Amphoteric
Answer : (c) Basic
(iv) The acid which is least volatile is:
(a) Hydrochloric acid
(b) Nitric acid
(c) Dilute sulphuric acid
(d) Concentrated sulphuric acid
Answer : (d) Concentrated sulphuric acid
(v) The gas formed, when calcium bisulphite reacts with dilute HNO:
(a) Sulphur trioxide
(b) Hydrogen
(c) Sulphur dioxide
(d) Hydrogen sulphide
Answer : (c) Sulphur dioxide
(vi) The IUPAC name of formic acid:
(a) Propanoic acid
(b) Methanoic acid
(c) Ethanoic acid
(d) Butanoic acid
Answer : (b) Methanoic acid
(vii) The metallic oxide which when reacts with HCI forms salt and water
(a) Carbon monoxide
(b) Nitrous oxide
(c) Ammonium hydroxide
(d) Sodium oxide
Answer : (d) Sodium oxide
(viii) Vanadium pentoxide is used as a catalyst in the preparation of :
(a) Nitrogen gas
(b) Nitrogen dioxide gas
(c) Sulphur trioxide gas
(d) Carbon dioxide gas
Answer : (c) Sulphur trioxide gas
(ix) The Catalyst used for the conversion of Ethene to Ethane:
(a) Iron
(b) Nickel
(c) Cobalt
(d) Molybdenum
Answer : (b) Nickel
(x) Substance which helps to lower the fusion point of the mixture in Hall Heroult Process:
(a) Coke
(b) Concentrated sodium hydroxide
(c) Fluorspar
(d) Concentrated potassium hydroxide
Answer : (c) Fluorspar
Section B
(Attempt any three questions from this section)
Question 2 :
(i) Define :
(a) Isomerism- the phenomenon in which more than one compounds have the same chemical formula but different chemical structures. Chemical compounds that have identical chemical formulae but differ in properties and the arrangement of atoms in the molecule are called isomers. Therefore, the compounds that exhibit isomerism are known as isomers.
(b) Ores–Ores are a mixture of minerals: processed to produce an industrial mineral or chemically treated to produce one or more metals. The steel, aluminum, chromium, zinc, mercury, manganese, tungsten, and some copper ores are typically processed for just one element
(ii) Name the following :
(a) The property by which carbon links with itself to form a long chain.-Catenation
(b) The saturated hydrocarbon having general formula CnH2n-2— Alkyne
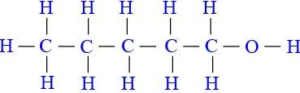
(iii) Draw the structural diagram of:
(a) pentanal
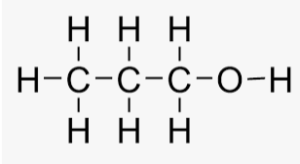
(b) propanol
(c) 2- butene
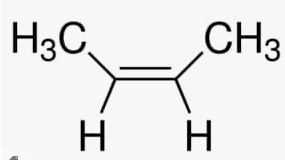
(iv) Complete and balance the following chemical equations:
(a) H2C = CH2 + Cl2 —( CCl4 / Inert solvent )—>
(b) C4H6 +O2 [excess) —>
(c) CH4 + O2 [excess| —>
Answer :
(a) CH2Cl−CH2Cl
(b) C4H6 + O2 = CO2 + H2O
(c) CH4 + 2 O2 → CO2 + 2 H2O
Question 3 :
(i) State the following :
(a) A compound formed when excess ammonia gas reacts with chlorine.
Ans-Ammonia and chlorine gas react to give Ammonium chloride and nitrogen gas
(b) A substance added to water, to manufacture sulphuric acid in Contact process
Ans- vanadium pentoxide
(ii) identity the gas P and Q in the reactions given below:
(a) A compound reacts with an acid to form gas P which has no effect on acidified K2Cr2O7 solution but tums lime water milky.
Ans- P is CO2
(b) A metallic nitrate reacts on heating gives oxygen gas along with a coloured gas Q.
Ans- NO2
(iii) State the observation for the following :
(a) Dry ammonia gas reacts with oxygen in the presence of a catalyst.
Ans-Reddish brown vapours of nitrogen dioxide are seen in the flask due to the oxidation of nitric oxide.
(b) Excess chlorine gas reacts with ammonia gas.
Ans-A yellow coloured highly explosive liquid.
(c) Carbon reacts with hot concentrated nitric acid.
Ans-form carbon dioxide, nitrogen dioxide and water
(iv) Write balanced equation for the following conversions:
(a) Carbon from cane sugar and concentrated sulphuric acid.
Ans-C12H22O11 (sugar) + H2SO4 (sulfuric acid) → 12 C (carbon) + 11 H2O (water) + mixture of water and acid
(b) Ferric nitrate from ferric hydroxide and nitric acid.
Ans-Fe(OH)3+3HNO3→Fe(NO3)3+3H2O.
(c) Ammonium sulphate from ammonium hydroxide and sulphuric acid.
Ans-2NH4OH + H2SO4—-> (NH4)2SO2+ 2H2O.
Question 4 :
(i) State the relevant reason for the following:
(a) Concentrated alkali is used for the concentration of bauxite ore.
Ans-Because it cause soluble Sodium meta aluminate and impurities remain insoluble.
(b) Fused alumina is reduced to aluminum by electrolysis.
Ans-since Alumina is highly stable.
(ii) State one use of the given alloys:
(a) Magnalium— Aircraft parts
(b) Duralumin– truck wheels, screw machine products,
(iii) Complete the table given below which refers to the Laboratory preparation off Ammonia gas:reactant use to prepare
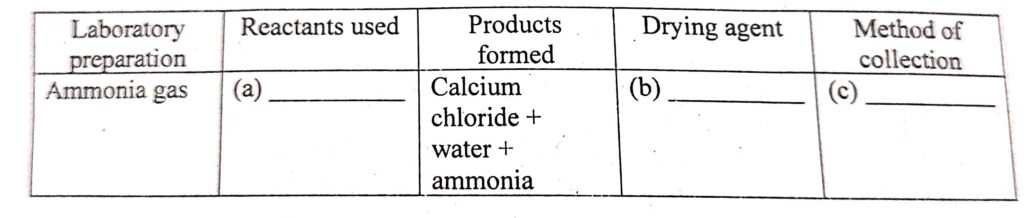
Ans (a) Reactants: Ammonium chloride (NH4Cl) and calcium hydroxide [Ca(OH)2] , (b) fresh quicklime or soda lime, (c)in an inverted dry gas jar by the downward displacement of air.
(iv) identify the terms for the following:
(a) The process used to purify Alumina by electrolytic reduction.—–Heroult process
(b)The experiment used to demonstrate the high solubility of HCI gas.—Fountain experiment
(c) The chemical property of sulphuric acid to form two types of salts with an alkali.—dibasic.
Question 5:
(i) Write the balanced chemical equation for the following:
(a) Action of heat on manganese dioxide and concentrated hydrochloric acid.
Ans–MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
(b) Zinc reacts with dilute hydrochloric acid to form zinc chloride.
Ans–Zn+2HCl→ZnCl2+H2↑
(ii) Select the right answer from the brackets and complete the statements:
In electrolysis of fused Alumina, the anode is made of (a)..graphite …….. [gas carbon/graphite] and the product formed at cathode is (b) ..oxygen … [oxygen / aluminum]/
(iii) Give the IUPAC name for the following :
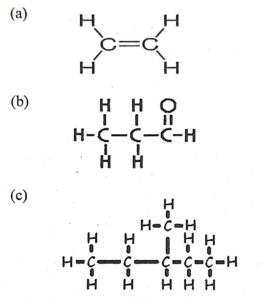
Ans — a) ethine , b) propenol, c) 2 methyl pentane
(iv) Study the diagram, which shows the brown Ring Test and answer the questions given below :
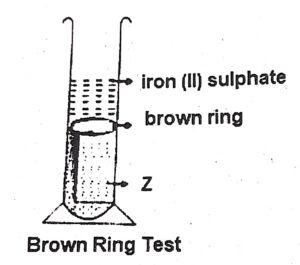
(a) Which ion is determined by Brown Ring Test?—nitrate ion
(b) Why is freshly prepared iron[II] sulphate used in the test?
Ans- because on exposure to the atmosphere, it is oxidized to ferric sulphate which will not give the brown ring
(c) Name the substance Z.–Sulphuric acid + nitric acid
Question 6:
(i) Distinguish between the following as directed:
(a) Sodium sulphite solution and sodium sulphate solution.
[using dilute H2SO4]
Ans- Sodium sulphate is dissolved in water and barium chloride solution is added, an insoluble white precipitate of barium sulphate is obtained. Sodium sulphite is warmed with dilute sulphuric acid, a colourless gas with a pungent and suffocating smell is evolved
(b) Lead salt solution and zinc salt solution.
Ans- Lead salt gives chalky with precipitates of lead hydroxide with ammonium hydroxide. This precipitates are insoluble in excess. Zinc salt gives gelatinous with precipitates of zinc hydroxide with ammonium hydroxide. This precipitates are soluble in excess
[using NH4OH solution in excess]
(i) Give one word for the following statements:
(a) The compounds of various metals found in nature with earthly impurities—Minerals
(b) A homogeneous mixture of two or more metals or a metal and a non-metal in specific ratios.–An alloy
(i) Identify the acid in each case:
(a) The acid formed when Sulphur reacts with concentrated nitric acid.—Sulphuric acid(H2SO4),
(b) An acid, which on adding to lead nitrate solution produces a white precipitate which is soluble on heating-
Ans- sulphonic acid (if in soluble on heating ) otherwise question may be wrong
(c) The acid formed when potassium nitrate reacts with a least volatile acid– form nitric acid
(iv) Match column A with column B:
| Name (A) | Functional group (B) |
| 1. Aldehyde | a) -OH |
| 2 Carboxylic acids | b)-CHO |
| 3. Alcohol | c)-COOH |
Answer :
| Name (A) | Functional group (B) |
| 1. Aldehyde | a) -OH |
| 2 Carboxylic acids | c)-COOH |
| 3. Alcohol | a) -OH |