Matter ICSE Class-6th Concise Selina Physics Solutions Chapter-1. We Provide Step by Step Answer of Objective, True False , Fill in the blanks, Match the following , Short / Long Answer Type , Numericals of Exercise-1 Matter . Visit official Website CISCE for detail information about ICSE Board Class-6.
Matter ICSE Class-6th Concise Selina Physics Solutions Chapter-1
A. Objective Questions
1. Write true or false for each statement
(a) The molecules of each substance are identical.
Answer. False
(b) The inter-molecular forces are effective at all distances between the two molecules.
Answer. False
(c) The molecules in a substance are in random motion.
Answer. True
(d) In a gas, the molecules can move anywhere in space. .
Answer. True
(e) The liquids are less viscous than the gases.
Answer. False
2. Fill in the blanks
(a) All the molecules of a substance are ….
(b) The inter-molecular spacing is … in solids … in liquids and…. in gases.
(c) The molecular motion in liquid and gas is in … path.
(d) In a solid, the molecules …. but they remain at their fixed positions.
(e) The inter-molecular forces are the weakest in …
(f) A solid exerts pressure …..
(g) The gases are ….dense.
(h) A solid is … rigid.
Answer
(a) All the molecules of a substance are identical.
(b) The inter-molecular spacing is least in solids more in liquids and still more in gases.
(c) The molecular motion in liquid and gas is in zig-zag path.
(d) In a solid, the molecules vibrate to and fro but they remain at their fixed positions.
(e) The inter-molecular forces are the weakest in gases.
(f) A solid exerts pressure downwards on its base.
(g) The gases are least dense.
(h) A solid is most rigid.
3. Select the correct alternative
(a) The diameter of a molecule is approximately
- 1 cm
- 10 cm
- 10-10 m
- 1 m
Answer
10-10 m
(b) The inter-molecular forces are strongest in
- solids
- liquids
- gases
- both (i) and (ii)
Answer
solids
(c) The molecules
- in solid, liquid and gas, move freely anywhere.
- in a solid, move freely within its boundary.
- in a liquid, move within its boundary.
- in a gas, move only within its boundary.
Answer
in a liquid, move within its boundary.
(d) The solids are
- more dense
- less dense
- least dense
- highly compressible
Answer
- more dense
(e) The inter-molecular forces in liquids are
- as strong as in solids
- stronger than in solids
- weaker than in solids
- weaker than in gases
Answer
weaker than in solids
4. Match the following columns

B. Short/Long answer questions
Question 1.
Define matter. What is its composition ?
Answer:
Matter is defined as anything which occupies space and has mass. It can be perceived by our sense of smell, touch, sight, hearing and taste.
Matter is composed of tiny particles known as atoms.
Question 2.
Name the three states of matter.
Answer:
The three states of matter are solids, liquids and gases.
Solids —A solid has a definite shape and definite volume.
Example – wood, stone, iron, ice etc.
Liquid —A liquid has a definite volume but not definite shape.
Example — water, juice, milk, oil, etc.
Gases —A gas neither has definite shape nor a definite volume.
Example – air, hydrogen, oxygen, watervapour etc.
Question 3.
What is a molecule ?
Answer:
The smallest unit of matter which can exist independently is called molecule.
Example: Oxygen molecule (O2) made up of two (O) atoms.
Question 4.
What is the approximate size of a molecule ?
Answer:
Matter is made up of molecules which are very small in size (~10-9 m).
Question 5.
One litre of water has 6.02 × 1026 molecules. Estimate the size of a molecule.
Answer:
The size of a particle (or molecule of matter is very small. 1 litre of water has 6.02 × 1026 molecules, so the volume of a particle of
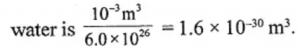
Thus the diameter of a water molecule is nearly 1.27 × 10-9 metre.
Question 6.
What do you mean by inter-molecular spacing ?
Answer:
Intermolecular space — The space between any two consecutive molecules of a substance is called intermolecular space
Question 7.
Describe a simple experiment to illustrate the existence of inter-molecular spacing.
Answer:
Take 100 ml of water in a measuring cylinder. Add 20 gram of salt in water gently and stir it well so as to dissolve the salt well in water. It is noticed that the level of water does not change. It shows that the particles of salt occupy spaces between the particles of water.
Question 8.
What do you mean by inter-molecular forces ?
Answer:
Intermolecular force of attraction — The force of attraction between the molecules (like molecules or unlike molecules) is called intermolecular force of attraction.
Question 9.
What are the forces of cohesion and adhesion ?
Answer:
The force of attraction between the molecules of similar kind is called force of cohesion.
Example: The forces between water molecules.
This force of cohesion keep the molecules of the substance bind together.
The force of attraction between different types of molecules is called force of adhesion.
Example: When a glass filled with water is emptied some water I particles remain stuck to the glass due to the adhesion between water molecules and glass.
Question 10.
State three characteristics of molecules of matter.
Answer:
The particles of matter called molecules, have the following characteristics:
- They are vety small in size.
- They have spaces between them.
- They are in constant random motion.
- They always attract each other.
Question 11.
State the approximate spacing between two molecules of a matter.
Answer:
The spacing between particles of a matter is called inter-molecular space.
Question 12.
How do the solids, liquids and gases differ in their following properties
(a) Size
(b) Shape
(c) Density
Answer:

Question 13.
The molecules in a substance are in motion. What type of path do they follow ?
Answer:
The particles in a substance are not at rest (in motion),and they move randomly in all possible directions in a zig-zag Path
Question 14.
Describe a simple experiment to illustrate that molecules are not at rest, but they constantly move.
Answer:
Take a beaker. Fill it partly with water. Add some lycopodium powder in the beaker containing water. Stir the contents of the beaker with a glass rod. Take out few drops of this suspension on a glass plate. Place it on the table and illuminate it with a table lamp. Observe the glass plate through a microscope. It is found that the fine particles of lycopodium powder move rapidly in a random manner and their path is zig zag as shown in figure below.
Question 15.
Write down five general properties of solids, liquids and gases.
Answer:
Solids:
- The molecules here are very tightly packed having negligible or veiy less intermolecular space.
- They have the strongest intermolecular force of attraction.
- The molecules have very small vibration about their mean position i.e. small amplitude.
- They have a definite shape and volume.
- They are generally hard and rigid.
- They are good conductors of heat.
Liquids:
- Molecules are less tightly packed.
- The intermolecular force of attraction is less than that of solids.
- The molecules here can move from one place to another
- Do not have any particular shape of their own and thus acquire the shape of the vessel.
- A particular quantity of a liquid has a definite volume at a given temperature.
Gases :
- The force of attraction between the molecules is the least.
- The intennolecular space is the largest.
- Neither have a definite shape nor a defmite volume.
- The molecules move independently.
- Worst conductors of heat.
Question 16.
Give the molecular model for a solid and use it to explain why a solid has a definite volume and a definite shape.
Answer:
The molecules are very tighty packed that there is no or very less intermolecular space and there is high intermolecular force of attraction (force of cohesion).
The molecules do not move about their mean position and thus solids have a definite shape and volume.
Question 17.
Describe the molecular modcl for a liquid. I-low does it explain that a liquid has no definite shape, but has a definite Volume ?
Answer
The molecules are less tightly packed as compared to solids and also there is lesser force of intermolecular attraction. The intermolecular distance is greater than that in the solids. Thus, they donot have a definite shape but acquire the shape of the vessel in which they are contained but have a definite volume at a given temperature.
Question 18.
A gas has neither a definite volume nor a definite shape. Describe the molecular model to explain it.
Answer:
Here the molecules are far apart from each other i.e. have the greatest intermolecular distance which result into the weakest intermolecular forces of attraction. The molecules as are not
bound by any strong force move about freely and thus gases do not have a definite shape and also do not have any definite volume.
Question 19.
Distinguish between the three states of matter—solid, liquid and gas on the basis of their molecular models.
Answer:
The molecules are very tighty packed that there is no or very less inteimolecular space and there is high intermolecular force of attraction (force of cohesion).
The molecules do not move about their mean position and thus solids have a definite shape and volume.
The molecules are less tightly packed as compared to solids and also there is lesser force of intermolecular attraction. The intermolecular distance is greater than that in the solids. Thus, they donot have a definite shape but acquire the shape of the vessel in which they are contained but have a definite volume at a given temperature.
The molecules are far apart from each other i.e. have the greatest intermolecular distance which result into the weakest intermolecular forces of attraction. The molecules as are not bound by any strong force move about freely and thus gases do not have a definite shape and also do not have any definite volume.
Question 20.
Distinguish between solids, liquids and gases on the basis of their following properties :
(a) compressibility
(b) fluidity
(c) rigidity
(d) expansion on heating
Answer:
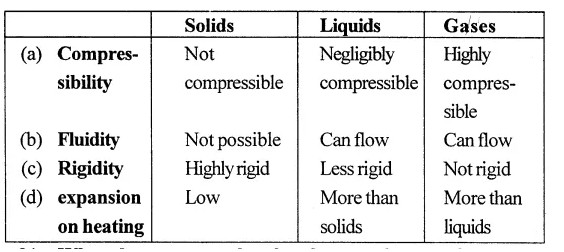
Question 21.
What do you mean by the change of state of matter ? Explain:
(a) the change of a solid into a liquid at a constant temperature, and
(b) the change of a liquid into a gas at a constant temperature.
Answer:
The change in state of matter of a substance from solid to liquid or from liquid to gas is brought by imparting heat energy to it at a constant temperature.
(a) The process of change of a substance from solid state into its liquid state on absorption of heat at a particular temperature, called the melting point, is called melting or fusion i. e
![]()
(b) The process of change of a substance from a liquid state to its gaseous state at a particular temperature, called the boiling point,
is called boiling or vaporisation, i.e.
![]()
Return to ICSE Class-6 Concise Selina Solutions
thanks
Please, Share with your friends



It help me very much and 19 answer was very lenghty.
update soon