Nitric Acid Dalal Simplified ICSE Chemistry Class-10 Solutions. Dalal Simplified ICSE Chemistry Nitric Acid by Dr Viraf and J Dalal for Class 10. Step by step Solutions of Nitric Acid by Dr Viraf and J Dalal of Concept of Simplified Dalal ICSE Chemistry.
Nitric Acid Dalal Simplified ICSE Chemistry Class-10
Get Other Chapter Dalal Simplified ICSE Chemistry Class-10 Solutions
How to Solve Mole Concept ICSE Chemistry Class-10
Note:– Before viewing Solutions of Nitric Acid by Dr Viraf and J Dalal Simplified ICSE Chemistry Solutions . Read the Nitric Acid Carefully to understand the concept in better way .After reading the Nitric Acid solve all example of your text book with ICSE Specimen Sample Paper for Class-10 Exam of Council. Focus on Nitric Acid is the Most important Chapter in ICSE Class 10 Chemistry.Previous Year Solved Question Paper for ICSE Board
Additional Question Nitric Acid Dalal Simplified ICSE
Question 1.
state how atmospheric nitrogen converts itself to nitric acid.
Ans.
-
- During lightning discharge, nitrogen in the atmosphere reacts with oxygen to form nitric oxide and further to nitrogen dioxide.
- The nitrogen dioxide dissolves in atmospheric moisture forming nitric acid.
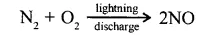 2NO + O2 →2NO2
2NO + O2 →2NO2
4NO3+2HO + O2→ 4 HNO3 (acid Rain)
Question 2
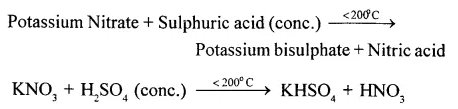
Give a word equation and balanced molecular equation for the laboratory preparation of nitric acid from (1) KN03 (2) NaNO3.
Answer:
(1)
(2)
Sodium Nitrate + Sulphuric acid (conc.) Sodium Bisuiphate + Nitric acid
NaNO3 + H2S04 (conc.) NaHSO4 + HNO3
Question 3.
In the laboratory preparation of nitric acid from – KNO3 or NaNO3 State
- The acid used
- The type of apparatus used
- The precautions to be taken during the preparation
- The method of collection of the acid
- The method of identification of the product i.e. acid formed.
Answer:
- Cone, sulphuric acid
- Glass retort
- Precautions are:
- Use all glass apparatus with no wooden or rubber cork.
- Control the temperature carefully at nearly 200 °C.
- Concentrated nitric acid vapours – condense and are collected in the water-cooled receiver.
- The vapours obtained in the receiver on heating alone or with copper turnings evolve – reddish brown fumes of nitrogen dioxide which turns acidified ferrous sulphate solution brown – proving that the vapours are of nitric acid.
Question 4.
Give reasons for the following – pertaining to the above laboratory preparation of nitric acid
Question 4(1).
concentrated hydrochloric acid is not used as a reactant in the laboratory preparation.
Answer:
Cone. HCl is not used as a reactant in the laboratory preparation of nitric acid. It is due to the following reasons:
- HCl is a volatile acid.
- HNO3, if formed, will oxidise HCl to Cl,. In the process, HNO3 will get reduced to NO or NO,. This very little yield, if any, of HNO, will be obtained and that too will be contaminated with HCl.
Question 4(ii).
The complete apparatus in the laboratory preparation does not contain parts made of rubber or cork.
Answer:
The complete apparatus is made of glass only – since the vapours of nitric acid being highly corrosive and attack rubber, cork, etc.
Question 4(iii).
The reaction temperature is maintained below 200°C
Answer:
The reaction temperature is maintained below 200°C. This is because at higher temperatures, HNO3 decomposes to give NO2. The brown coloured NO2 dissolves in HNO3 to give it a yellow colour. Thus, if the temperature is allowed to go beyond 200°C, the product (HNO3) obtained is not pure (colourless).
Question 4(iv).
At high temperatures the sodium sulphate or phtassium sulphate formed, forms a crust and sticks to the glass apparatus.
Answer:
Formation of a hard residual crust of the corresponding sulphate [Na2SO4 or K2SO4] which being a -poor conductor of heat, sticks to the glass and cannot be easily removed from the apparatus.
Question 5.
State the colour of
(1) pure nitric acid
(2) nitric acid obtained in the laboratory
(3) nitric acid obtained in the laboratory after passage of air or addition of water to it.
Answer:
- Pure nitric acid is colourless.
- Nitric acid obtained in laboratory is pale yellow in colour.
- The pale yellow colour of nitric acid disappears and hence it becomes colourless.
Question 6.
State which reaction of ammonia forms the first step of Ostwald’s process.
Answer:
The first step of Ostwald’s process involves catalytic oxidation of ammonia to nitric oxide and water (steam).
![]()
Question 7.
Convert ammonia to nitric acid by the above process giving all conditions.
Answer:
Step I
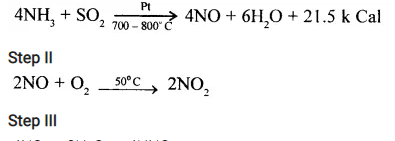
4NO2 + 2H2O → 4HNO3
Question 8.
State how —
- a higher ratio of the reactant air
- exothermicity of the catalytic reaction
- use of low temp, in the conversion of NO to NO2 – affects each related step in Ostwald’s process.
Answer:
- Excess of air carries the reactions in forward direction as oxygen is needed in all the three reactions, leading to the formation of nitric acid.
- The exothermicity of catalytic reaction helps in stopping external heating, there by saving on energy.
- Low temperature (less than 50°C)
Question 9.
State why nitric acid
- Stains the skin
- Cannot be concentrated beyond 68% by boiling.
Answer:
- Nitric acid combines with protein of the skin forming a yellow compound Xanthoproteic acid, stains skin yellow.
- It is because at 68% concentration it forms a constant boiling mixture, i.e., if heated beyond this concentration then proportion of water vapour and nitric acid vapour, leaving the dilute acid does not change. Thus, it cannot be concentrated by boiling.
Question 10.
State two conditions which affect the decomposition of nitric acid.
Answer:
The conditions which affect the decomposition of nitric acid are:
- Presence of sunlight
- Higher temperature.
Question 11.
State the change in colour of pure concentrated nitric acid on initial and prolonged decomposition.
Answer:
Yellowish brown colour is changed to dark yellowish brown colour on prolonged decomposition.
Question 12.
State the cation responsible for turning moist neutral litmus red on reaction with dil. HNO3.
Answer:
Hydrogen |H+| ions and Nitrate ions.
Question 13.
State why nitric acid is a strong oxidising agent and yields varying products such as NO, NO2 on reaction with metals,non-metals etc.
Answer:
The oxidising property of nitric acid is based on the fact that when nitric acid undergoes decomposition, it yields nascent oxygen, which is very reactive.
2HNO3 (cone.) → H2O + 2NO2 + [O]
2HNO3 (dil.) → H2O + 2NO + 3[O]
This nascent oxygen oxidises metals, non-metals, organic and inorganic compounds. During the process, nitric acid itself gets reduced to various products (NO, NO2, N2O, NH3, etc.) depending upon the concentration of the acid, reaction temperature and activity of the metal with which it is reacting.
Question 14.
Give an equation for reaction of cone. HNO3 with
(1) carbon
(2) copper.
Answer:
- C + 4HNOs → CO2 + 2H2O + 4NO2
- 3Cu + 8HNO3 → 3Cu (NO3)2 + 4H2O + 2NO
Question 15.
Convert nitric acid to sulphuric acid using a non-metal.
Answer:
S + 6HNO3 → H2SO4 + 2H2O + 6NO2
Question 16.
State how you would obtain
(1) Hydrogen
(2) Nitric oxide
(3) Nascent chlorine – from nitric acid. State the concentration of nitric acid used in each case.
Ans.
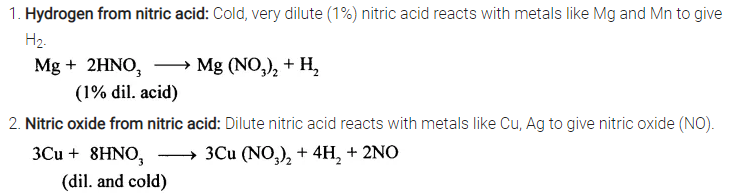
- Hydrogen from nitric acid: Cold, very dilute (1%) nitric acid reacts with metals like Mg and Mn to give H2.
- Nitric oxide from nitric acid: Dilute nitric acid reacts with metals like Cu, Ag to give nitric oxide (NO).
- Nascent chlorine from nitric oxide: A mixture of cone, nitric acid (I part) and cone, hydrochloric acid (III parts) (by volume) reacts with noble metals like gold and platinum. In this reaction, nascent chlorine is formed as an intermediate.
HNO3 (cone.) + 3HCl (cone.) → 2H2O + NOCL + 2|Cl|
Question 17.
State why hydrogen is liberated when zinc reacts with dil.HCl but not with dil. HNO3.
Answer:
Zinc displace hydrogen from dil. HCl.
Zn + 2HCl(dil.) → ZnCl2 + H2
However, when zinc reacts with dil HNO3, no hydrogen is obtained. This is because nitric acid is a strong oxidising agent. Nitric acid oxidises the hydrogen produced to water and hence no hydrogen is liberated.
Question 18.
State a reason for the inactivity of iron and aluminium on reaction with fuming HNO3.
Answer:
Pure or fuming nitric acid renders metals like iron (Fe) and Al- passive i. e., inactive. This is due to the formation of a thin oxide coating on the surface of the metal which prevents further action.
Question 19.
State your observation when
(1) nitric acid is added to saw dust
(2) cone, nitric acid is heated
(a) in absence of copper
(b) in presence of copper.
Answer:
- Nitric acid being a strong oxidising agent decomposes to give nascent oxygen, which being very reactive, oxidises organic compounds to carbon dioxide and water. Saw dust is organic in nature. When hot cone. HNO3 is poured over saw dust, it burst into flames due to oxidation.
- (a)
When cone. HNO, is heated, it decomposes to give brown coloured pungent smelling gas nitrogen dioxide (NO2).
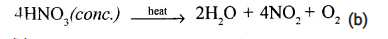
(b) When. ”cone. HNO3 is heated in the presence of copper, brown coloured, pungent smelling NO2 is formed alongwith blue coloured copper nitrate.
Cu + 4HNO(cone.)→ Cu(NO3)2 + 2H2O + 2NO2
Question 20.
State how addition of nitric acid to acidified FeSO, serves as a test for the former.
Answer:
Nitric acid oxidises iron(II) sulphate to iron (III) sulphate with the liberation of nitric oxide gas.
6FeSO4 +3H2SO4 + 2HNO3 (dil. ) → 3Fe2(SO4)3 + 4H2O +2NO
The nitric oxide so formed reacts wtih more of iron(II) sulphate to form nitrosoferrous sulphate, which appears in the form of brown ring at the junction of liquids.
FeSO4 + NO → FeSO4.NO
Question 21.
Name three chemical products manufactured from nitric acid. Give two general uses of HNO3.
Answer:
- Three chemical products manufactured from nitric acid. Explosives (T.N.T., picric acid, nitrocellulose etc.)
- Fertilizers (Ammonium nitrate, calcium ammonium nitrate or C.A.N.)
- Dyes (Picric acid and other nitro dyes)
Two general uses of nitric acid
- For refinning of noble metals like gold, platinum etc.
- For etching on stainless steel.
–Try Also :–