Organic Chemistry Class-10 Goyal Brothers ICSE Solutions Ch-11. Step by Step Solutions of Exercise and Objective Type Questions of Goyal Brothers Prakashan Chapter-11 Organic Chemistry for ICSE Class 10 .
Organic Chemistry – Test , use, observation seen , preparation , properties (physical and chemical) of Organic Compound explain with suitable chemical reaction. Visit official Website CISCE for detail information about ICSE Board Class-10 Chemistry .
Organic Chemistry Class-10 Goyal Brothers ICSE Solutions Ch-11
-: Select Topics :-
Exercise 1 Page –191
Organic Chemistry Class-10 Goyal Brothers ICSE Solutions Ch-11
Question 1. What is vital force theory? Why was it discarded?
Answer :Vital Force Theory is a theory made by the Scientist Berzelius in 1809 which assumed that organic compounds are only formed in living cells and it is impossible to prepare them in laboratories.
Question 2. Name the scientist who disproved the vital force theory.
Answer : vital force theory was discarded because Friedrich Wohler showed that it was possible to obtain an organic compound (urea) in the laboratory
Question 3. What do you understand by the following terms?
(i) Organic chemistry
(ii) Organic compounds
(iii) Catenation
Answer :
(i) Organic chemistry–Organic chemistry is the study of the structure, properties, composition, reactions, and preparation of carbon-containing compounds, which include not only hydrocarbons but also compounds with any number of other elements, including hydrogen (most compounds contain at least one carbon–hydrogen bond), nitrogen, oxygen
(ii) Organic compounds– Organic compound, any of a large class of chemical compounds in which one or more atoms of carbon are covalently linked to atoms of other elements, most commonly hydrogen, oxygen, or nitrogen. The few carbon-containing compounds not classified as organic include carbides, carbonates, and cyanides
(iii) Catenation- Catenation is the binding of an element to itself through covalent bonds to form chain or ring molecules. Examples: Carbon is the most common element that exhibits catenation. It can form long hydrocarbon chains and rings like
Question 4. What are the unique properties of carbon?
Answer :
Unique properties of carbon
1) Catenation: It is the ability to form bonds with other carbon atoms. 2) Tetravalency: Due to 4 valency of carbon, it is capable of bonding with four other atoms.
3) A carbon atom can bond with another carbon atom two or three times to make double and triple covalent bonds between two carbon atoms
Question 5. Why are there very large number of organic compounds?
Answer :Carbon forms large number of compound due to following reasons: 1. Catenation: The unique property of the ‘C’ element to be able to form continuous links with other ‘C’ atoms through covalency called catenation, is one reason for the existence of a large number of organic compounds
Question 6. Distinguish between organic and inorganic compounds on the basis of solubility.
Answer : Generally, organic compounds are less soluble in water than inorganic compounds. Organic compounds are more inflammable (more volatile) but are poorer conductors of heat and electricity than inorganic compounds
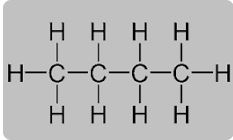
Question 7. With reference to butane, explain what do you understand by the following terms:
(i) Molecular formula
(ii) Condensed formula
(iii) Structural formula
Answer :
(i) Molecular formula–C4H10
(ii) Condensed formula – CH3-CH2-CH2-CH3.
(iii) Structural formula –
Question 8. Name three major classes of aliphatic hydrocarbon.
Answer :Aliphatic hydrocarbons are divided into three main groups according to the types of bonds they contain:
1. alkanes,
2.alkenes,
3. alkynes
Question 9. How do paraffins differ from unsaturated hydrocarbons?
Answer :Alkanes – Are saturated hydrocarbons that therefore contain only hydrogen and carbon atoms bonded to each other, and typically follow the chemical formula CnH2n+2. A common example is paraffin. 2. Alkenes – These unsaturated hydrocarbons are molecules that contain at least one carbon-to-carbon double bond
Question 10. What do you understand by alkyl group? How are they formed?
Answer :Alkanes can be described by the general formula CnH2n+2. An alkyl group is formed by removing one hydrogen from the alkane chain and is described by the formula CnH2n+1. The removal of this hydrogen results in a stem change from -ane to -yl
Question 11. What is functional group? Give definition with example.
Answer :Functional groups are collections of atoms that attach the carbon skeleton of an organic molecule and confer specific properties. .and Functional groups include: hydroxyl, methyl, carbonyl, carboxyl, amino, phosphate, and sulfhydryl.
Question 12. identify and name the functional groups of the following organic compounds
(i) C2H5OH
(ii) CH3Cl
(iii) CH3CHO
(iv) CH3CH2COOH
(v) CH3COCH3
(vi) HCOOH
Answer :
(i) C2H5OH– Alcohol
(ii) CH3Cl– Alkyl Halide
(iii) CH3CHO– Aldehyde
(iv) CH3CH2COOH– Carboxylic Acid
(v) CH3COCH3– Ketone
(vi) HCOOH– Carboxylic Acid
Exercise 2 Page –197
Organic Chemistry Class-10 Goyal Brothers ICSE Solutions Ch-11
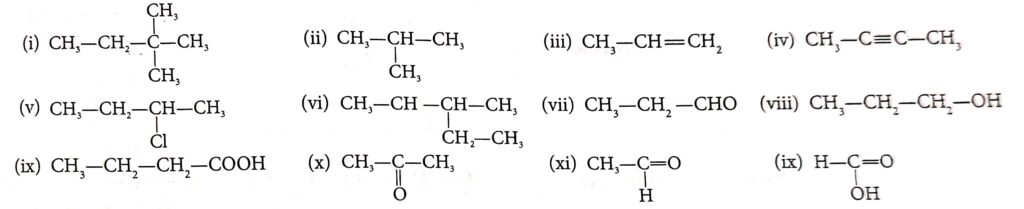
Question 1. Write the IUPAC name of the following compounds:
Answer :
(i) 2 ,2 Di Methyl Butane
(ii) 2-methyl Propane
(iii) Prop-1-ene
(iv) Butan-2-yne
(v) 2, Chloro-butane
(vi) 3-methyl pentane
(vii) propanal
(viii) propanol
(ix) butanoic acid
(x) propanone
(xi) ethenal
(xii) methenoic acid
Question 2. Write the structural formulae for the following compounds:
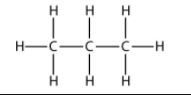
(i) Hexane
(ii) 2-methyl butane
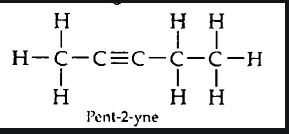
(iii) Pent-2-yne
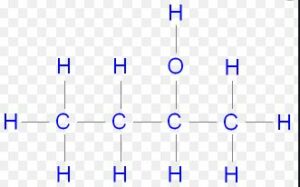
(iv) Butan-2-ol
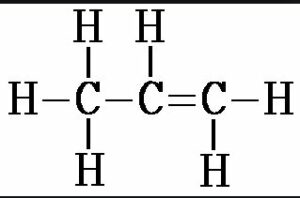
(v) Prop-1-ene
(vi) Formic acid
(vii) Acetic acid
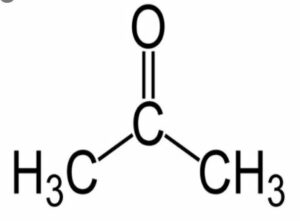
(viii) Acetone
Answer :
structural formulae
(i) Hexane– CH3- CH2- CH2- CH2- CH2- CH3
(ii) 2-methyl butane
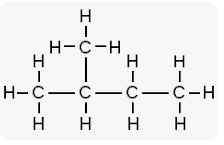
(iii) Pent-2-yne
(iv) Butan-2-ol
(v) Prop-1-ene
(vi) Formic acid
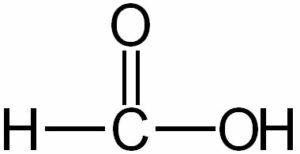
(vii) Acetic acid
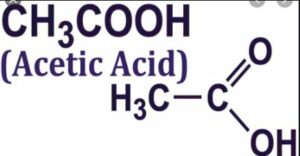
(viii) Acetone
Question 3. Give the IUPAC name, molecular formula, condensed formula, general formula and structural formula of the following hydrocarbons:
(i) Propane
(ii) n-Butene
(iii) Ethene
Answer :
(i) Propane—
IUPAC name-Alkane
molecular formula- C5H12
condensed formula- CH3-CH2-CH2-CH2-CH2-CH3
general formula -CnH 2n+2
structural formula-.
(ii) n-Butene—
IUPAC name, –Alkine
molecular formula,= C4H8
condensed formula,–CH2=CH2-CH2-CH2-CH3
general formula –CnH 2n
structural formula
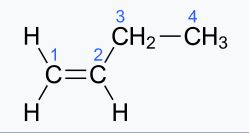
(iii) Ethene—
IUPAC name,–Ethene
molecular formula,-C2H4
condensed formula,-CH2=CH2
general formula –CnH 2n
structural formula-
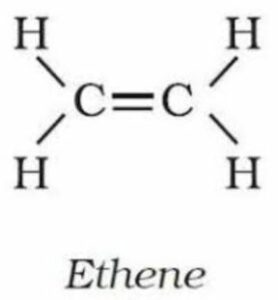
Question 4. What do you understand by the term homologous series? Give four characteristics of homologous series.
Answer :
Characteristics are : The members of the homologous series have same functional group. Members have the same general formula. Members have the almost same chemical properties due to same functional group. Members have common general method of preparation and deffer in molecular mass by CH2 (14amu)
Question 5. Give names and molecular formulae of first four homologous of alkynes and alcohols.
Answer :
alkynes — ethyne (C2H2), propyne (C3H4), butyne (C4H6)
alcohols.– methenol (CH3OH) , ethenol (C2H5OH), propenol (C3H7OH)
Question 6. What do you understand by isomerism?
Answer : Isomerism, the existence of molecules that have the same numbers of the same kinds of atoms (and hence the same formula) but differ in chemical and physical properties
Question 7. Draw chain isomers of the following-
(i) C5H12
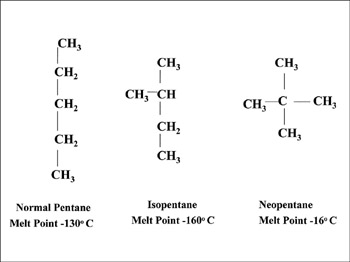
(ii) C6H14
Hexane has five isomers:
- Hexane, CH3CH2CH2CH2CH2CH3, a straight chain of six carbon atoms.
- 2-Methylpentane (Isohexane), CH3CH(CH3)CH2CH2CH3, a five-carbon chain with one methyl branch on the second.
- 3-Methylpentane, CH3CH2CH(CH3)CH2CH3, a five-carbon chain with one methyl branch on the third.
- 2,3-Dimethylbutane, CH3CH(CH3)CH(CH3)CH3, a four-carbon chain with one methyl branch on the second and third.
- 2,2-Dimethylbutane (neohexane), CH3C(CH3)2CH2CH3, a four-carbon chain with two methyl branches on the second.
Question 8. Draw position isomers of the following:
(i) C4H8
(ii)C5H8
Answer:
(i) C4H8
But-1-ene
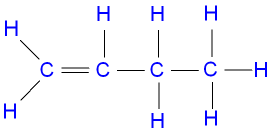
cis-But-2-ene
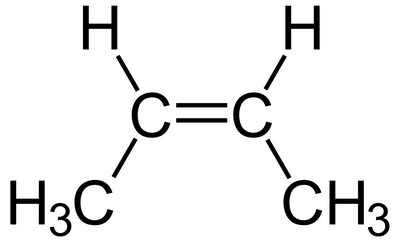
(ii)C5H8
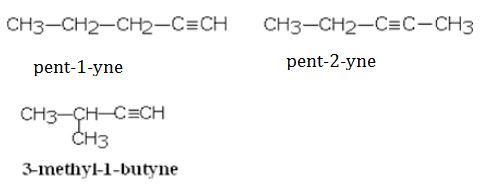
Question 9. What are the general properties of homologous series?
Answer :Features include a general formula and neighbouring members differing by CH2, with similar chemical properties and with a gradation in physical properties. Homologous series are ‘families’ of organic compounds. They share common characteristics: They all contain the same functional group
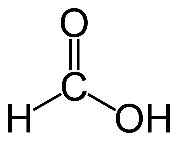
Question 10. Write the name and structural formula of the simplest organic acid.
Answer :
Simplest organic acid is Formic acid and the chemical formula is .
Exercise 3 Page –203
Organic Chemistry Class-10 Goyal Brothers ICSE Solutions Ch-11
Question 1. How is methane gas prepared in the laboratory?
Answer : In the laboratory, methane is formed by heating sodium ethanoate with a mixture of sodium hydroxide and calcium oxide, called soda lime, on heating in the presence of a catalyst, calcium oxide, the -COONa group from sodium ethanoate is replaced by the hydrogen atom from sodium hydroxide, forming methane and sodium hydroxide gets converted into sodium carbonate
Question 2. What do you understand by the term substitution reaction?
Answer : Substitution reaction, any of a class of chemical reactions in which an atom, ion, or group of atoms or ions in a molecule is replaced by another atom, ion, or group.
Question 3. How does excess chlorine react with :
(i) methane
(ii) ethane?
Answer : excess chlorine react with :
(i) methane—When a mixture of methane and chlorine is exposed to ultraviolet light – typically sunlight – a substitution reaction occurs and the organic product is chloromethane. However, the reaction doesn’t stop there, and all the hydrogens in the methane can in turn be replaced by chlorine atoms
(ii) ethane–Ethane reacts with chlorine by free radical halogenation in the presence of sunlight when chlorine breaks down to form two chlorine radicals. The chlorine radical reacts with ethane to give ethane radical which reacts with other chlorine to generate to give halogenated product C2Cl6
Question 4. How is ethane gas prepared in the laboratory?
Answer : Ethane gas can be prepared in a laboratory by the cracking of hydrocarbons such as kerosene or candle wax. … The vapor of kerosene when passes over heated porcelain pieces undergoes cracking. The gas so formed is collected in gas jars by the downward displacement of water. This gas is mostly ethane (ethylene)
Question 5. What do you understand by pyrolysis?
Answer : Pyrolysis is a thermochemical treatment, which can be applied to any organic (carbon-based) product. It can be done on pure products as well as mixtures. In this treatment, material is exposed to high temperature, and in the absence of oxygen goes through chemical and physical separation into different molecules
Question 6. How will you obtain ethane from ethyl iodide? Give chemical equation.
Answer :
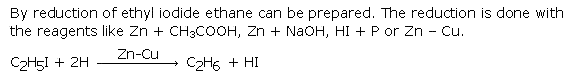
(i) Excess air
(ii) Insufficient supply of air
Answer : alkanes burn in:
(i) Excess air—However, these alkanes burn very rapidly. The combination of alkanes with oxygen generating heat is known as combustion. More precisely, combustion is defined as “a chemical reaction with oxygen in which alkane is converted into carbon dioxide and water with the release of heat energy”
(ii) Insufficient supply of air–It occurs when there is not enough amount of oxygen for fuel to react completely. This leads to the formation of carbon or carbon monoxide. Carbon monoxide formed as a by product is a colourless poisonous gas.
Methane + (little) Oxygen → Carbon + Water
CH₄ + O₂ → C + 2H₂O
Question 8. Write names and structural formulae of isomers of fourth and fifth homologous members of alkanes.
Answer :
The fourth member of the alkane series is butane .
The fifth member of the alkane series is pentane
Question 9. How will you bring about the following conversions?
(i) Methane to methyl alcohol
(ii) Methane to methanoic acid
(iii) Ethane to acetaldehyde
Answer : the following conversions
(i) Methane to methyl alcohol–Methane, is first converted into methyl chloride by chlorination, in the presence of diffused sunlight. Methyl chloride on hydrolysis forms methyl alcohol.

(ii) Methane to methanoic acid–The progressive oxidation of methane by 2 electron losses to form methanol, formaldehyde and ultimately formic acid using simple vanadium compound with the use of H2O2.
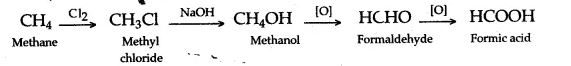
(iii) Ethane to acetaldehyde
The catalytic conversion of ethane to acetaldehyde has been achieved using nitrous oxide as the oxidant. The resulting ethyl radical reacts with surface MoO to produce a surface ethoxide, which may either decompose to ethylene or react further with surface OH− to form acetaldehyde or with water to form C2H5OH.
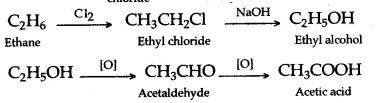
Question 10. Give two uses of
(i) Methane
(ii) Ethane
Answer : two uses of
(i)Methane— It is used primarily as fuel to make heat and light. It is also used to manufacture organic chemicals.
(ii)Ethane–The chief use of ethane is the production of ethene (ethylene) by steam cracking. … Ethane can be used as a refrigerant in cryogenic refrigeration systems. On a much smaller scale, in scientific research, liquid ethane is used to vitrify water-rich samples for electron microscopy
Exercise 4 Page –207
Organic Chemistry Class-10 Goyal Brothers ICSE Solutions Ch-11
Question 1. How will you prepare ethylene gas in the laboratory? Support your answer by chemical equations.
Answer :
Balanced Equation of ethylene:
CH3-CH2OH + H2SO4 → CH3-CH2HSO4+H2O
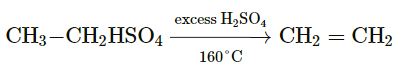
The gas is collected by downward displacement of water.
Question 2. How does ethylene react with (i) bromine (ii) alkaline potasium permanganate (iii) HCl gas?
Answer :
ethylene react with
(i) bromine — When bromine is passed through the inert solution of ethene, an addition reaction takes place with the formation of 1, 2, dibromoethane.

(ii) alkaline potasium permanganate— Ethene reacts with alkaline potassium permanganate solution to form glycol.

(iii) HCl gas— Ethene reacts with HCl to form ethane glycol monochloride.

Question 3. State two uses of ethylene gas.
Answer : Uses of ethylene :
(i) It is used in the manufacture of polyethylene which is a valuable plastic.
(ii) It is used in the artificial ripening of fruits.
Question 4. Write the structural formula of isomers of the following: (i) Pentene (ii) Butene
Answer : isomers of the following:
(i) Pentene -It’s chemical formula is C5 H10. There are three isomers of pentene: 1-pentene, with the double bond at carbon 1. cis-2-pentene, with the double bond at carbon 2, and the functional groups on the same side
(ii) Butene-The chemical formula for butene is: C4 H8, which means it’s made up of four carbon atoms and eight hydrogen atoms. The ‘-ene’ part of the name refers to an alkene, so we know that butene’s structure must include a carbon double bond
- but-1-ene
- but-2-ene)
- 2-methylprop-1-ene
Question 5. Write the chemical equation for the preparation of ethylene gas by dehydrohalogenation with alcoholic potash.
Answer : preparation of ethylene gas by dehydrohalogenation with alcoholic potash.: Alcohols reacts with concentrated sulphuric acid which results in the formation of alkenes due to the elimination of a water molecule. As water molecule is removed in this reaction, it is called as acidic dehydration of alcohol and the dehydrating agent is concentrated sulphuric acid.
Question 6. Why does ethene give addition reactions?
Answer :Ethene and bromine are an addition reaction because ethene is an alkene – it has a double bond. It is easier for new atoms to open the double bond and react there than to remove the hydrogen already attached, and then bond to it, which would be a substitution reaction
Question 7. What do you understand by hydrogenation? Give the necessary conditions and chemical equation for the hydrogenation of ethene.
Answer : Hydrogenation is a chemical reaction between molecular hydrogen and other compounds and elements. Hydrogenation is used in many applications such as the food industry, petrochemical industry and the pharmaceutical manufacturing industry
The hydrogenation of ethene
Ethene reacts with hydrogen in the presence of a finely divided nickel catalyst at a temperature of about 150°C. Ethane is produced.
![]()
![]()
Question 8. What do you mean by polymerization? How can polyethene be prepared by ethene?
Answer :
Polymerization, any process in which relatively small molecules, called monomers, combine chemically to produce a very large chainlike or network molecule, called a polymer. The monomer molecules may be all alike, or they may represent two, three, or more different compounds.
![]()
Question 9. How will you bring about the following conversion?
(i) Ethene into ethyl alcohol (ii) Ethene into acetaldehyde
Answer : conversion
(i) Ethene into ethyl alcohol–Ethanol is manufactured by reacting ethene with steam. The reaction is reversible, and the formation of the ethanol is exothermic. Only 5% of the ethene is converted into ethanol at each pass through the reactor
(ii) Ethene into acetaldehyde–The catalytic conversion of ethane to acetaldehyde has been achieved using nitrous oxide as the oxidant. Water vapor is essential for high selectivity to acetaldehyde.
Question 10. Give molecular formula and names of first four homologous members of alkenes.
Answer :
| Name | Number of Carbon atoms | Molecular Formula CnH2n |
|---|---|---|
| ethene | 2 | C2H2(2) = C2H4 |
| Propene | 3 | C3H2(3) = C3H6 |
| Butene | 4 | C4H2(4) = C4H8 |
| Pentene | 5 | C5H2(5) = C5H10 |
Question 11. Starting from ethylene, how will you obtain acetic acid?
Answer – ethylene is oxidized into acetaldehyde with a PdCl2–CuCl2 catalyst, which is subsequently oxidized to acetic acid with a manganese acetate catalyst.
Exercise 5 Page –211
Organic Chemistry Class-10 Goyal Brothers ICSE Solutions Ch-11
Question 1. How is acetylene gas prepared in the laboratory?
Answer :Acetylene is prepared in the laboratory from calcium carbide. Calcium carbide is decomposed to acetylene with water as per the following reaction. Acetylene is collected in a glass jar by the downward displacement of wate
Question 2. How does acetylene gas react with chlorine?
Answer :The chlorine gas reacts with the acetylene (as well as many other hydrogenated compounds). This reaction is not what one might first expect, since it is not the product of the addition of the halogen across the carbon-carbon triple bond. The product is formed by the abstraction of hydrogen from the hydrocarbon
Question 3. How will you distinguish between alkanes, alkenes and alkynes?
Answer :
Alkanes have single bonds between carbons in a hydrocarbon. Saturated hydrocarbons are saturated with hydrogen and are the simplest. They are represented in general as CnH2n+2 in case of non-cyclic structures or straight-chain structures. They are also called paraffins. In alkanes, there are four bonds for each carbon atom; it could be either C-H or C-C bond. Each hydrogen atom has to be bonded with a carbon atom. The simplest alkane is CH4. Alkane compounds are not very reactive; this is because the carbon bonds are stable and do not break easily. They have no functional groups attached to the carbon atoms
.Alkenes are unsaturated hydrocarbons which have at least one double bond. They are represented as CnH2n in general when there is no other functional group. They are also called olefin or olefine . they are more reactive than alkanes but relatively stable as compared to alkynes.
Alkynes are also unsaturated hydrocarbons; they have one or more triple bonds between the carbon atoms. Their general formula is CnH2n-2, in the case of any non-cyclic compound. They are also known as acetylenes. Alkynes are more reactive than alkenes and alkanes; They are highly reactive due to the presence of triple, unsaturated bonds and readily undergo addition reactions
Question 4. Write names and structural formulas of isomers of third and fourth homologous members of alkynes.
Answer :
Chain isomerism
Alkynes having or five or more carbon atoms show isomerism due to different structures of the carbon chain.
Functional isomerism
Alkynes are functional isomers of dienes i.e. compounds containing two double bonds.
CH3-CH2-C≡CH But-1-yne
CH2=CH-CH=CH2 But-1,3-dienes
CH2=C=CH-CH3 But-1,2-diene
Question 5. How does acetylene gas react with alkaline KMn04?
Answer :Ethyne or acetylene on reaction with cold KMnO4 adds 4 OH− to the triple bond. It is oxidised by a dilute aqueous solution of potassium permanganate to form oxalic acid.
Question 6. Write four uses of acetylene gas.
Answer :The welding process that uses acetylene is known as oxy-fuel cutting or gas cutting. This method is used to cut or weld materials that require temperatures as high as 3,500 °C (6,330 °F). Among all other gases, acetylene is capable of producing the hottest flame
Question 7. What is the characteristic chemical reaction for unsaturated hydrocarbon?
Answer :Addition reaction is a characteristic of unsaturated hydrocarbons like alkenes and alkynes. An unsaturated hydrocarbon combines with another substance to produce a single product (saturated hydrocarbon), in the presence of a catalyst
Question 8. How will you distinguish between saturated and unsaturated hydrocarbons on the basis of chemical reaction?
Answer : Bromine water test – is used to differentiate between the unsaturated compounds (like alkenes and alkynes) and the saturated compounds. For this purpose, bromine is used in the form of bromine water. A solution of bromine in water is called bromine water. Bromine water has a red-brown color due to the presence of bromine in it. When bromine water is added to an unsaturated compound, then bromine gets added to the unsaturated compound and the red-brown color of bromine water is discharged. So, if an organic compound decolorizes bromine water, then it will be an unsaturated hydrocarbon (containing a double bond or a triple bond), but saturated hydrocarbon (alkanes) do not decolorize bromine water
Exercise 6 Page –216
Organic Chemistry Class-10 Goyal Brothers ICSE Solutions Ch-11
Question 1. State four industrial uses of alcohol.
Answer : four industrial uses of alcohol.
- Alcoholic Drinks.
- Industrial methylated spirits.
- Use of ethanol as a fuel.
- Ethanol as a solvent.
- Methanol as a fuel.
- Methanol as an industrial feedstock.
Question 2. What is spurious liquor? What makes it harmful?
(a) What is the common name of the first member of the aldehyde group of compounds?
(b) State the IUPAC name and the structural formula of the above compound.
Answer : Spurious liquors include: illicit liquor (un-authorized preparation, not fit for human consumption and not complying with the BIS standards) and denatured alcohol (prepared for industrial uses and is rendered entirely unfit for human consumption by adding denaturants).
(a) the common name of the first member of the aldehyde group of compounds: formaldehyde,
(b) the IUPAC name – Methanal
the structural formula – HCHO
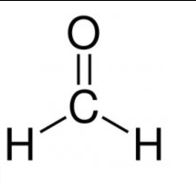
Question 3. Complete the following chemical equation :
CH3COOH + C2H5OH —–conc. H2SO4—–>
Answer :
CH3COOH + C2H5OH —–conc. H2SO4—–> CH3COOC2H5 +H2O
ii) C2H5OH———-Conc.H2SO4————–>CH2=CH2 + H2O
443k
Question 4. Give the IUPAC name of CH3COOH.
Answer : Ethenoic Acid
Question 5. An organic compound A has a molecular formula C2H402 and is acidic in nature. On heating with ethyl alcohol and conc. sulphuric acid, vapours with pleasant fruity smell are given out. What is the compound A and what is the chemical equation involved in this reaction?
Answer :
The compound ‘A’ with molecular formula C2H4O2 is ethanoic acid (acetic acid). Upon heating with ethanol (ethyl alcohol) and concentrated sulphuric acid, ethyl ethanoate (ethyl acetate) is formed as the product. It is an ester with pleasant or fruity smell. The reaction is known as esterification reaction
CH3COOH (ethanoic acid (acetic acid) + C2H5OH ethanol (ethyl alcohol) —→ CH3COOC2H5 (ethyl ethanoate (ethyl acetate) + H2O
Question 6. Fill in the blank space with a suitable word:
The organic acid present in vinegar is ………. .
Answer :
The organic acid present in vinegar is …acetic acid……
Question 7. An organic compound A has a molecular formula C2H6O. On oxidation in air in the presence of heated copper as catalyst, it is oxidised to CH3COOH. What is compound A? Give the equation for the reaction.
Answer :
The compound A’ on oxidation gives acetic acid, CH3COOH. Now, acid is obtained by the oxidation of an alcohol or an aldehyde, so compound A should be an alcohol (ethyl alcohol) or an acetaldehyde because its molecule contains two carbon atoms. Ethyl alcohol is CH3CH2OH or C2H6O and acetaldehyde is CH3CHO or C2H4O
CH3CH2OH ——— CH3COOH.
Question 8. Write the chemical formulae of ethanoic acid and methanal.
Answer :
the chemical formulae of ethanoic acid is –CH3COOH
Write the chemical formulae of methanal.–HCHO
Question 9. Write two tests to demonstrate that acetic acid (CH3COOH) is acidic in nature.
Answer :
Test to show that CH3COOH is acidic are :
- When litmus test is done, it turns blue litmus red.
- It react with bases to form salt and water.
Question 10. White the name and structural formula of the simplest organic acid.
Answer : Simplest organic acid is Formic acid and the chemical formula is HCOOH
Question 11. What do you understand by the term decarboxylation?
Answer :Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain.
Question 12. How will you convert ethanoic acid to methane?
Answer :Ethanoic acid can be coverted to methane by decarboxylation i.e. ethanoic acid on reaction with NaOH gives sodium acetate, which on evaporation and heating leads to decarboxylation giving methane
Objective Type Questions
Page – 217 to 219
Organic Chemistry Class-10 Goyal Brothers ICSE Solutions Ch-11
I. Multiple Choice Questions
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
II. Fill in the blanks spaces with the choice given in brackets:
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
- ………………..
III Choose from the following list, as what matches the descriptions given below :
[Isomers, ethene, esterification, diastase, zymase, ethyne, calcium carbide, ethanol, hydrogen, ethene glycol]
1. An organic compound with -CHO group.
2. Onganic compounds which has same molecular formula, but different structural formulac.
3. An inonganic compound used in the preparation of acetylene gas.
4. A gas commonly used for ripening raw fruits
5 Agas which forms reddish precipitate when passed through ammoniacal cuprous chloride
6. Arcaction between alcohol and acetic acid in the presence of conc. sulphurc acid.
7. An enzyme which breaks dowm starch into maltose.
&. An eymes which converts glucose into alcohol.
9. The product formed when ethene gas is passed through 1% alkaline potassium permmanganate solution.
10. A gas liberated when ethanol is treated with sodium at room temperature.
–: End of Organic Chemistry Class-10 Goyal Brothers :–
Return of : Chemistry Class-10 Goyal Brothers Prakashan
Thanks
Share with your friends