Organic Chemistry Dalal Simplified ICSE Class-10 Solutions Chapter 8:- Solutions of Dr Dalal Simplified ICSE Chemistry Chapter-8 Organic Chemistry by Dr Viraf and J Dalal for Class 10. Step by step Solutions of Dr Dalal Simplified ICSE Chemistry by Dr Viraf and J Dalal of Organic Chemistry Dalal Simplified .
Organic Chemistry Dalal Simplified ICSE Class-10
Get Other Chapter Dalal Simplified ICSE Chemistry Class-10 Solutions
How to Solve Mole Concept ICSE Chemistry Class-10
Note:– Before viewing Solutions of Organic Chemistry by Dr Viraf and J Dalal Simplified ICSE Chemistry Solutions of Chapter-8 .Read the Chapter-8 Organic Chemistry Carefully to understand the concept in better way .After reading the Chapter-8 Organic Chemistry solve all example of your text book with ICSE Specimen Sample Paper for Class-10 Exam of Council. Focus on Numerical’s Problems. Organic Chemistry is the Most important Chapter in ICSE Class 10 Chemistry.Previous Year Solved Question Paper for ICSE Board
ADDITIONAL QUESTIONS
Question 1.
Explain the term ‘Organic Chemistry ’. State the ‘Natural sources ’ and ‘Importance’ of organic compounds.
Answer:
- Organic Chemistry-It is the chemistry of specific carbon compounds except – oxides, carbonates, bicarbonates and metallic carbides.
- Plants, Animals, Petroleum, dyes and drugs are all natural sources.
- Compounds of organic origin are : Food – carbohydrates, vitamins Dyes-azodyes Clothing – cotton, silk and wool Fuels – petrol Medicines – penicillin Explosives – trinitrotoluene.
Question 2.
Explain the ‘unique nature of carbon atom’ with specific reference and meaning to —
(a) ‘Tetravalency’ — leading to formation of single, double and triple bonds
(b) ‘Catenation’ — leading to formation of straight chain, branch chain and cyclic compounds.
Answer:
Some unique properties shown by carbon atom are :
(a) Tetravalency
(b) Catenation
(c) Ability to form multiple bonds.
(a) Tetravalency : Atomic number of carbon is 6. Its electronic configuration is 2, 4. Therefore, it has four electrons in its valence shell. Carbon atom can neither lose nor gain electrons to complete its octet (not possible from energy point of view). Therefore, carbon atom completes its octet by sharing four electrons with other atoms, i.e., it can form four covalent bonds, called its tetracovalency.
For example:
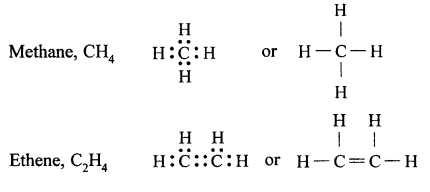
(b) Catenation: The property by virtue of which a large number of atoms of the same element get linked together through single or multiple covalent bonds, forming straight or branched chains and rings of different sizes, is called catenation. Carbon shows catenation to the maximum extent due to strong carbon-carbon bonds and its tetracovalency.
In this process of catenation, carbon atoms form straight or branched chains and cyclic rings of various sizes and can involve single, double or triple covalent bonds.
Question 3.
State reasons for ‘Justification of a separate branch’ for ‘Organic Chemistry.
Answer:
This is due to the following reasons:
- The number of known organic compounds is very large as compared to the number of known inorganic compounds.
- Organic compounds involve only a few elements (C, H, O, N, S, P, F, Cl, Br, I etc.), whereas inorganic compounds involve all the known elements.
- and Organic compounds have complex nature and have high molecular mass.
- compounds of carbon involve covalent bonds whereas inorganic compounds involve electrovalent bonds.
- Organic compounds show isomerism whereas inorganic compounds do not show isomerism.
- The properties of organic compounds are different from inorganic compounds.
All these facts convince us to study organic chemistry as a separate branch of chemistry.
Question 4.
State five differences between the characteristics of organic and inorganic compounds. State how organic compounds are classified.
Answer:
Characteristics of organic compounds :
- These are made up of only a few elements C, H, O, N, S,X(C1, Br,l)
- These involve covalent bonds.
- these are generally gases or liquids
- They have low melting and boiling points.
- and They are combustible.
- They show molecular reations.
- They show isomerism.
- These are non-conductors of electrocity.
- There are generally insoluble in water but soluble in organic solvents.
Characteristics of inorganic compounds :
- These are made up of all the known elements.
- These involve ionic bonds.
- and These are generally solids.
- They have high melting and boiling points.
- non-combustible.
- They don’t show isomerism.
- These are generally good conductors of electricity.
- These are generally soluble in water but insoluble in organic solvents.
(b) Classification of Organic Compounds Aliphatic – Open Chain Compounds
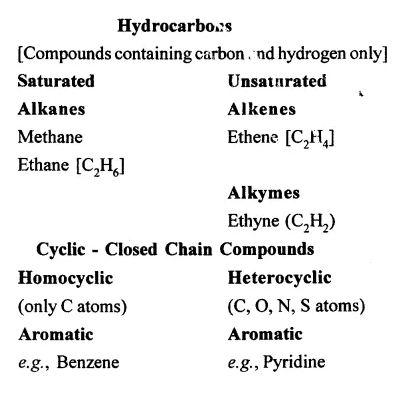
Question 5.
Explain the term ‘Homologous series’. State the general characteristics of members of the series with special reference to molecular mass or molecular formula.
Answer:
Homologous series is a series of organic compounds, that are grouped into a smaller number of series of compound.
General Characteristics of homologous series :
- The members of a series have same functional group.
- Two consecutive members of a homologous series differs each other in their composition
by – CH2unit
Example :
Alcohol (-OH)
CH3 – OH, CH3 – CH2 – OH, CH3 – CH2 – CH2 – OH - The members of a homologous series can be represented by same general formula.
Example :
Alcohol-CnH2n+1OH
Aldehyde — CnH2n+1|CHO
Carboyxlic acid — CnH2n+1COOH - The members of a particular homologous series have almost same chemical properties due to presence of same functional group.
- The physical properties (like solubility, melting point, boiling point, state) of members of a homologous series either gradually increase or decrease with increase in molecular mass.
- The members of a homologous series can be prepared by same or common general method of preparation.
- The first member of homologous series generally shows certain different chemical behavior than other members of the series.
Question 6.
Differentiate between — ‘Molecular formula’ and ‘Structural formula’ — of an organic compound. Write the ‘condensed structural formula and ‘branched structural formula’ of ethene.
Answer:
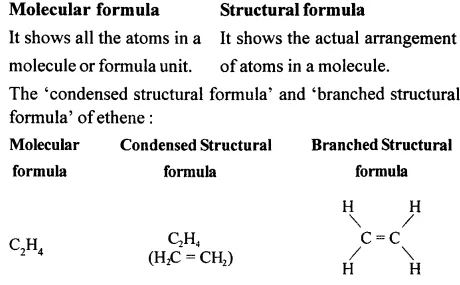
Question 7.
State what are ‘Alkyl groups ’. State the alkyl group of the parent alkane — methane and ethane.
Answer:
Alkyl Group : It is obtained by removing one hydrogen atom from a molecule of an alkane.
Methane : Methyl (Alkyl group)
Ethane : Ethyl (Alkyl group)
Question 8.
State what are ‘Functional groups’. Name the following functional groups —
…………..
X = -F, -Cl, -Br, -I ; -C=O; -C-O-C
Answer:
Functional Groups : An atom, radical or bond which defines the structure of an organic compound and give if its characteristic properties
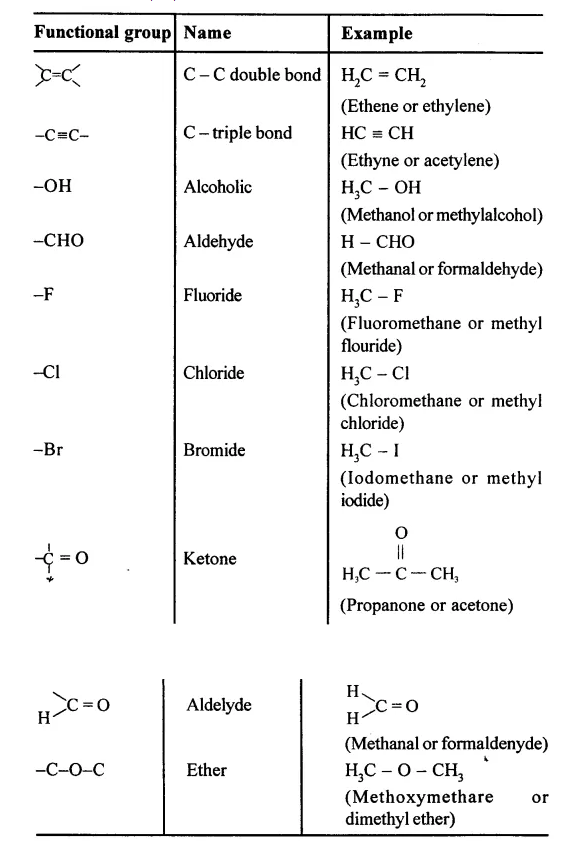
Question 9.
Explain the terms — ‘Isomers’ and ‘Isomerism’. State the ‘Characteristics of isomers’ with reference to —
Properties of isomers ; Number of isomers with relation to carbon atoms in the isomer.
Differentiate between — ‘Chain isomerism’ and ‘Position isomerism ’ – with suitable examples.
Answer:
‘Isomers’ and ‘Isomerism
Two or more compounds having the same molecular formula but different physical and chemical properties are called isomers and this phenomenon is known as isomerism.
Isomers have the same number of atoms of each element in them and the same atomic weight but differ in other properties. For example, there are two compounds with the molecular formula C2H6O. One is ethanol (ethyl alcohol), CH3CH2OH, a colorless liquid alcohol; the other is dimethyl ether, CH3OCH3, a colorless gaseous ether.
Alkanes with more than three carbon atoms form isomers. The various isomers differ in the framework of the carbon chains.
Differentiate between — ‘Chain isomerism’ and ‘Position isomerism’
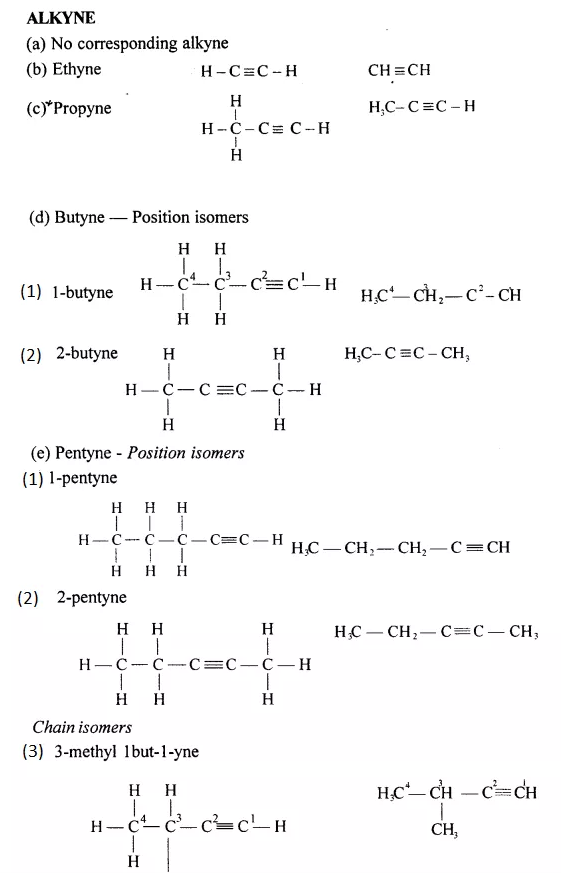
Chain isomers: Compounds having same molecular formula with difference in carbon chain pattern like linear or branch are called chain isomers. 1-Pentyne is chain isomer for 3-methyl Butyne.
CH3 – CH2 – CH2 – C = CH and CH3– C(CH3) – C = CH
Position isomers: Compounds having same molecular formula ^ with difference in position of the functional group are called position isomers. 1-Butyne and 2-Butyne are position isomers.
CH3– CH2– C=CH and CH3 – C = C – CH3.
Question 10.
Explain the term – ‘Nomenclature’. State its need with reference to organic compounds. State the basic rules of Nomenclature by the trivial system – with suitable examples. Explain the longest chain rule and the smallest number for functional groups rule of Nomenclature by the IUPAC system – with suitable examples.
Answer:
(a) Nomenclature :
Nomenclature is the system of assignment of names to organic compounds.
Need for Nomenclature : Very large number of organic compounds with varying molecular structure need a systematic method of nomenclature. Further many a times same molecular formula represents two or more compounds (isomerism).
(b) Nomenclature by Trivial System:
In this method, name of an organic compound is derived from its;
- Source (e.g., benzoic acid is obtained by distillation from gum benzoin, fructose or fruit sugar from fruits etc).
- Latin or Greek origin (e.g., formic acid, HCOOH is present in sting of red ants, formicus in Latin means an ant).
- Properties (e.g., palmitic acid is an acid derived from palm oil etc).
(c) Longest Chain Rule :
In the nomenclature of alkanes, the longest continuous chain if C-atoms is selected. For this, alkyl groups, if present, are written in the expanded form.
For example,
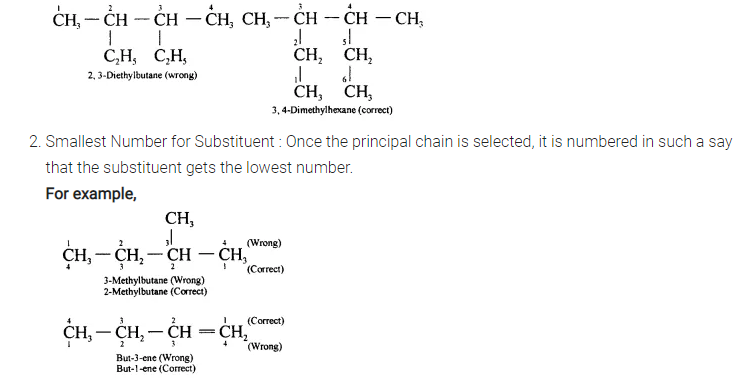
Question 11.
Explain the term – ‘Hydrocarbons’. State the two main groups of hydrocarbons with examples. Draw a chart differentiating — ‘Alkanes, Alkenes andAlkynes’ — with respect to:
- General formula
- Characteristic bond
- IUPAC and the common name of the first three members and condensed/branched/electronic structural formula of each
- Availability of electrons
- Reactivity
- Characteristic reaction.
Answer:
Hydrocarbons — They are aliphatic open chain organic compounds containing carbon and hydrogen only.
Molecular formula is CxHy where X and Y are whole numbers.
- Saturated hydrocarbons — Homologous series of alkanes.
- Unsaturated hydrocarbons — Series of alkynes and alkenes.
- General formula :
Alkanes — CnH2n+2
Alkenes — CnH2n
Alkynes — CnH2n_2
- Characteristic bond
Alkanes → C – C < single bond
Alkenes → C = C < double bond
Alkynes → C = C < triple bond - IUPAC Name Condensed/branched/electronic structural formula of each
- General formula :
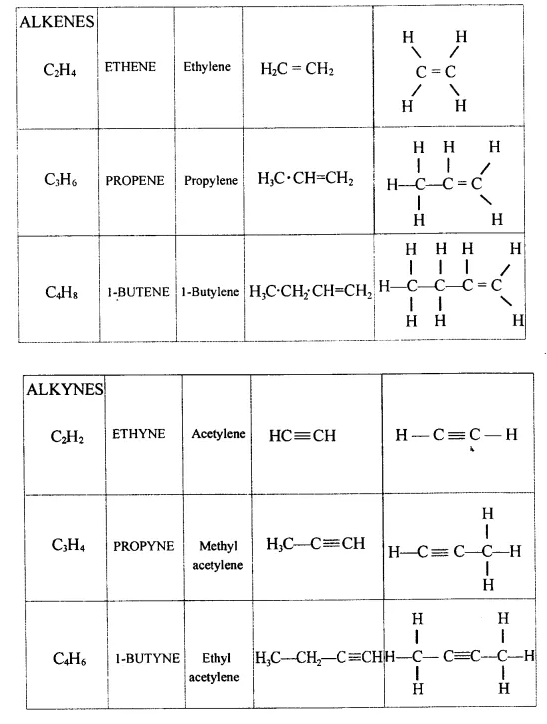
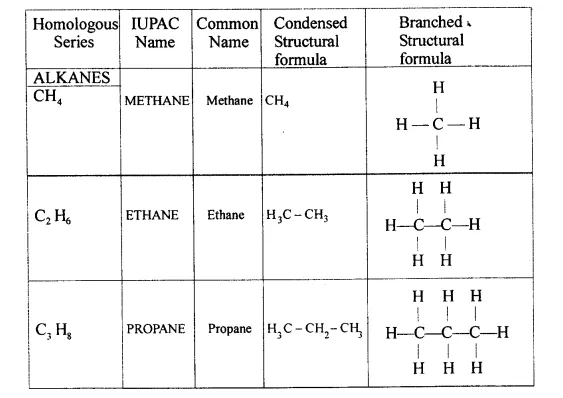
-
- Availability of electrons
Alkanes — Not available
Alkenes — Available Alkynes — Available - Reactivity
Alkanes — Less reactive
Alkenes — More reactive
Alkynes — Most reactive - Characteristic Reaction
Alkanes — Substitution reaction
Alkenes — Addition reaction
Alkynes — Addition reaction
- Availability of electrons
Question 12. Organic Chemistry Dalal Simplified
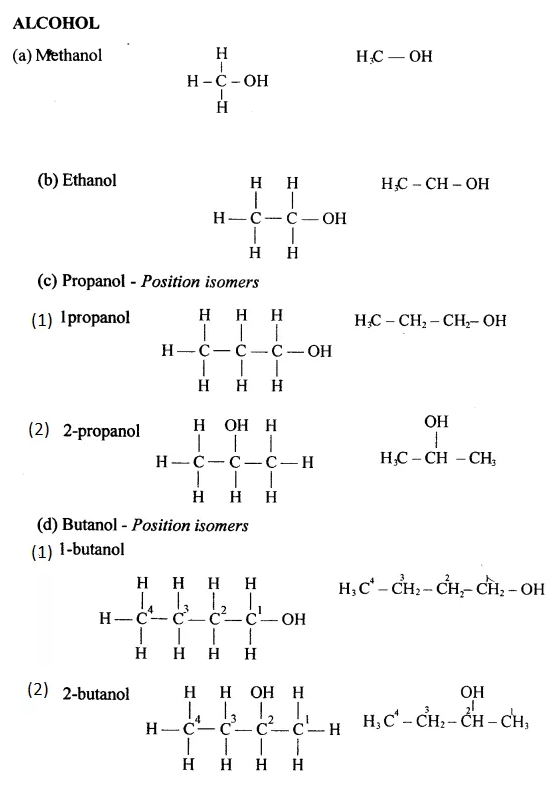
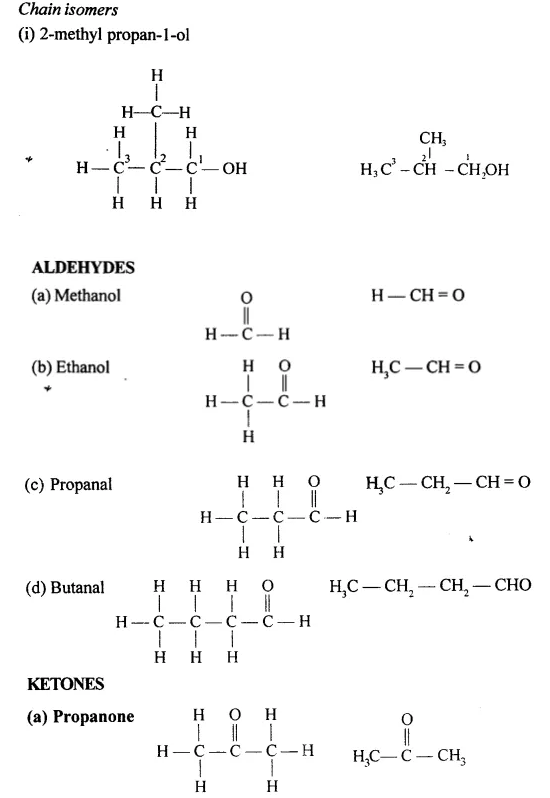
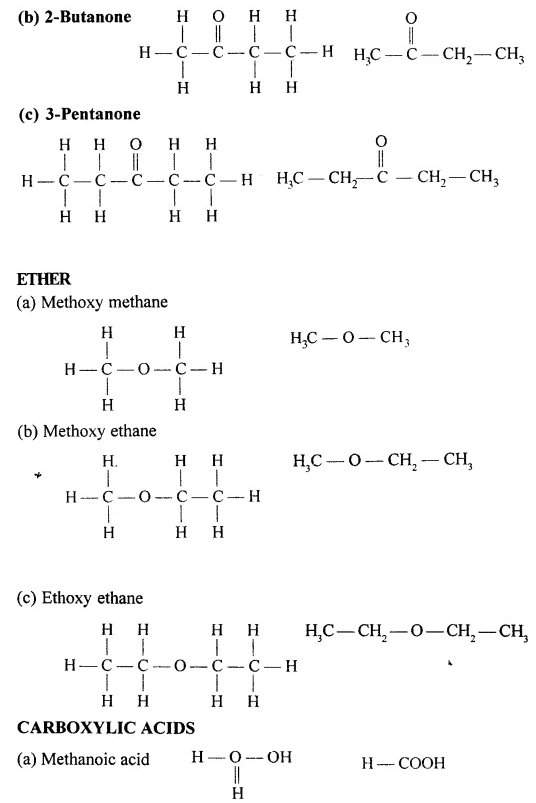
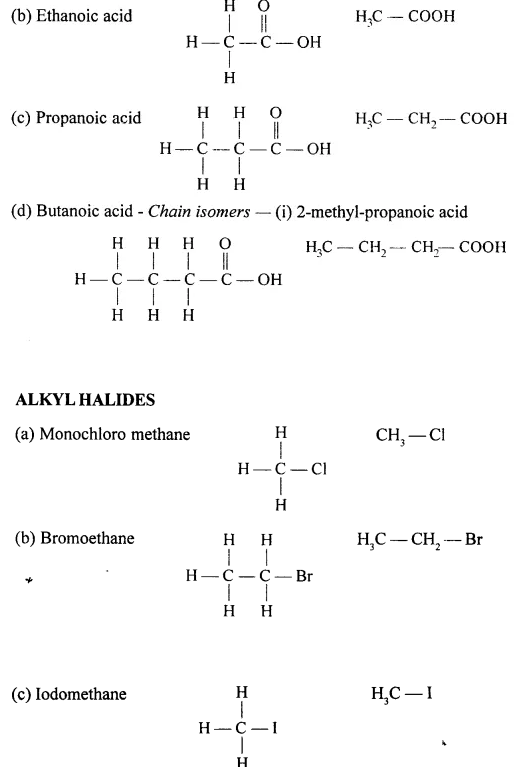
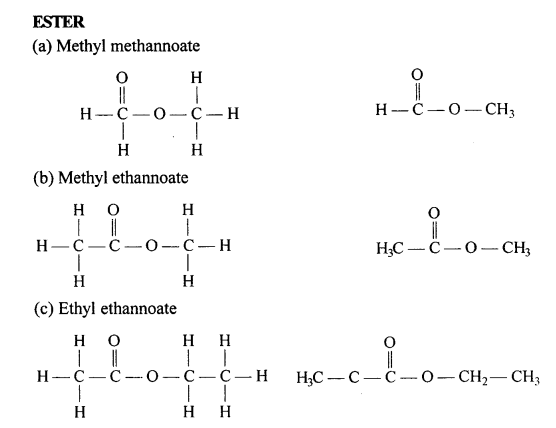
Draw the structural formula of each of the following :
…………………
Answer:
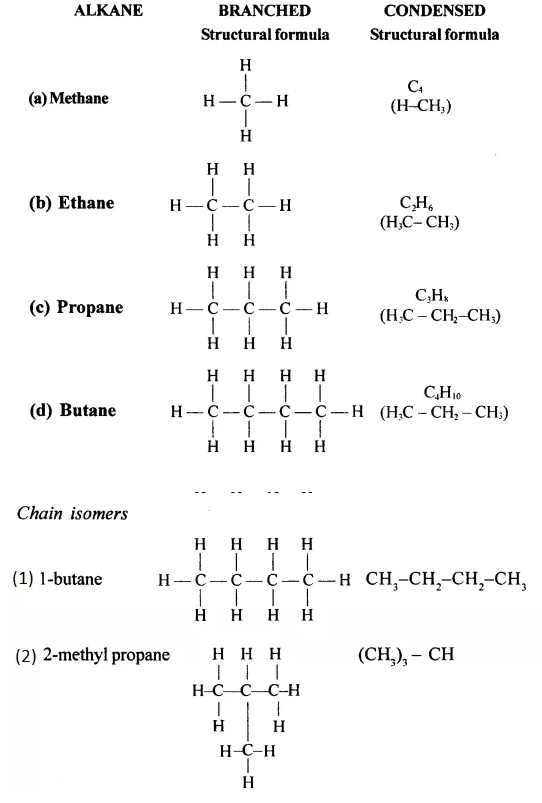
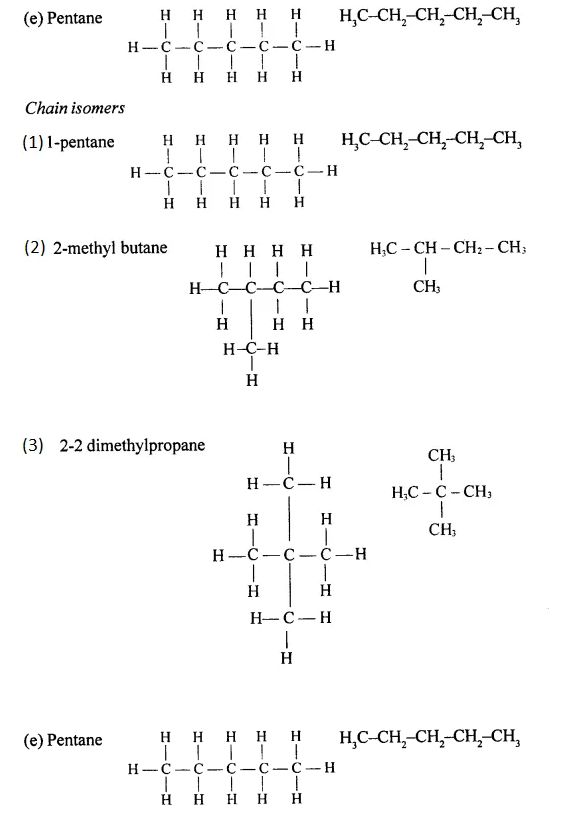
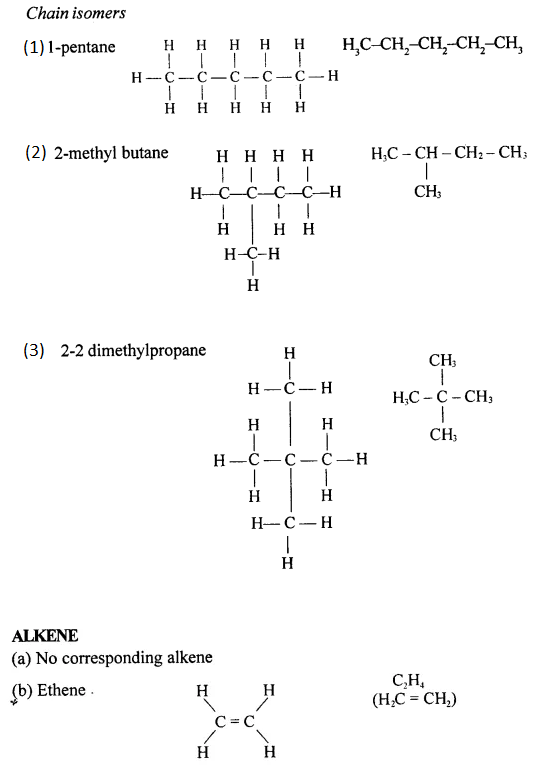
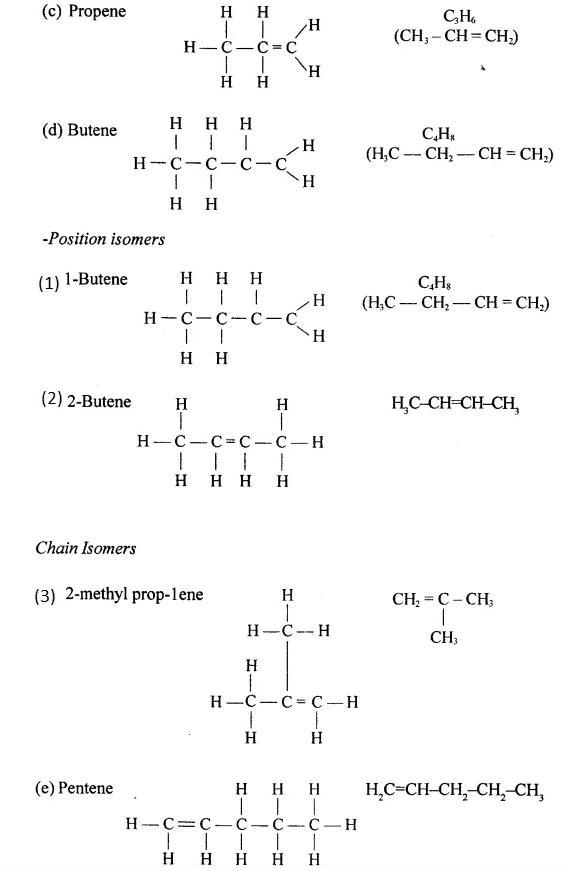
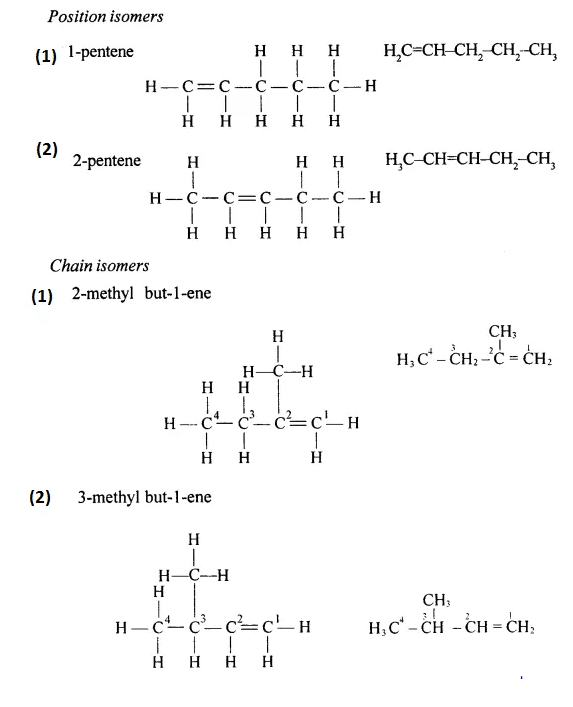
Question 13.
Give the IUPAC name of the compounds numbered (I) to (y).
…………….
Answer:
- Methyl butanol, 2-Methyl-1-butanol
- 2, 2-dimethyl propanol
- 2-Bromocyclo pentan- I -ol
- 3-Methylbutanal
- 3-Methyl-2-butanone
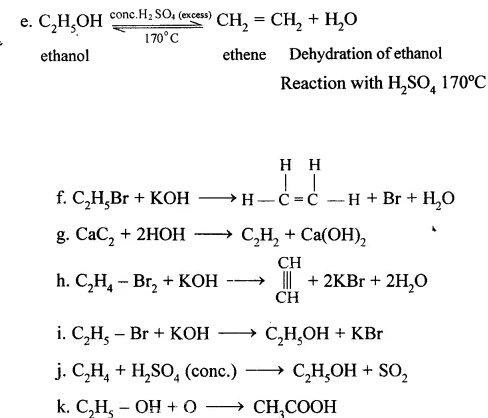
Question 14.
…………………
Answer:
(a) CH3COONa + NaOH → CH4 + Na2CO3
(b) CH3I + 2|H| → CH4 + HI
(c) C2H5COONa + NaOH → C2H6 + NaCO3
(d) C2H5Br + 2(H) → C2H6 + HBr
Question 15. Organic Chemistry Dalal Simplified
Give equations for the conversions of – Methane, Ethane, Ethene, Ethyne, Methanol, Ethanol and Ethanoic Acid.
……………
Answer:
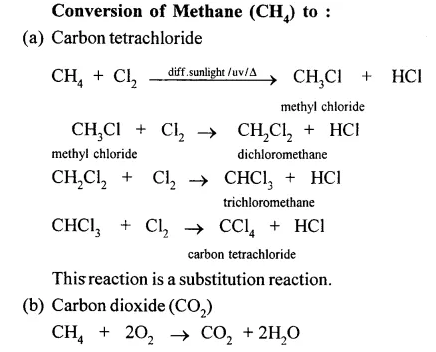
When methane is burnt in excess of air or oxygen with pale blue flame it gives carbon dioxide gas, water and heat energy. This reaction is complete oxidiation reaction.
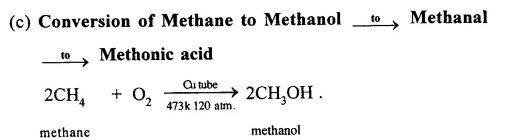
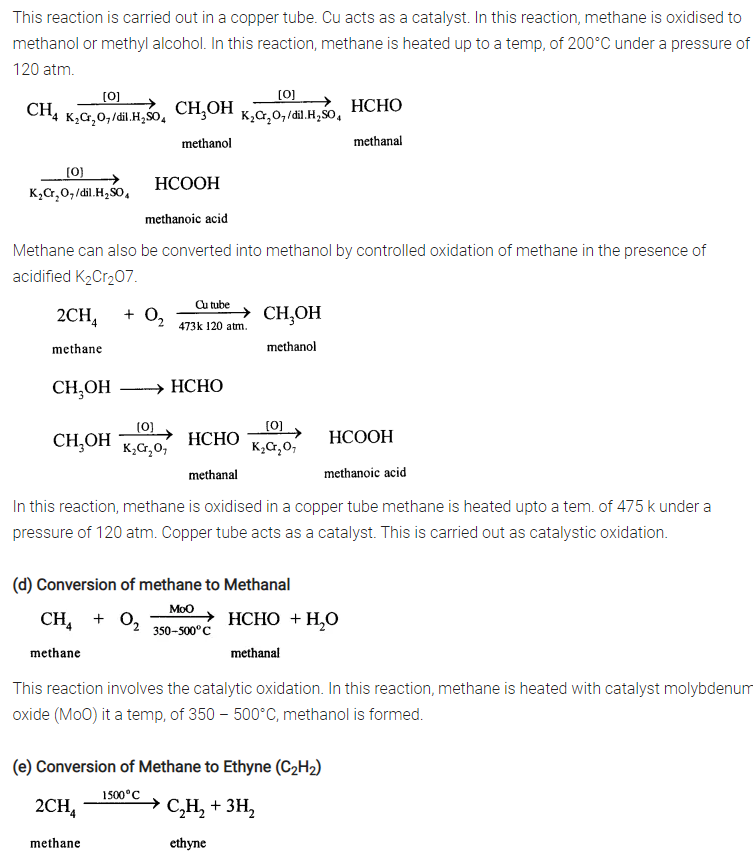
(e) Conversion of Methane to Ethyne (C2H2)
When methane is heated to about 1500°C in an electric arc and then suddenly cooled, the product is C2H2and Hydrogen.
Conversion of Ethane (C2H6) to
(a) Hexachloro ethane (C2Cl6)

C2H4Cl2 + Cl2 → C2H4Cl2 + HCl
and C2 H4Cl2 + Cl2→ C2H3Cl3 + HCl
so C2 H3Cl3 + Cl2 → C2H2Cl4 + HCl
therefore C2 H2Cl4 + Cl2→ C2HCl5 + HCl
C2HCl5 + Cl2 → C2Cl6 + HCl
This reaction is a substitution reaction.
(b) Carbon dioxide (CO2)
2C2H6 + 7O2 (excess) →4CO2 + 6H2O
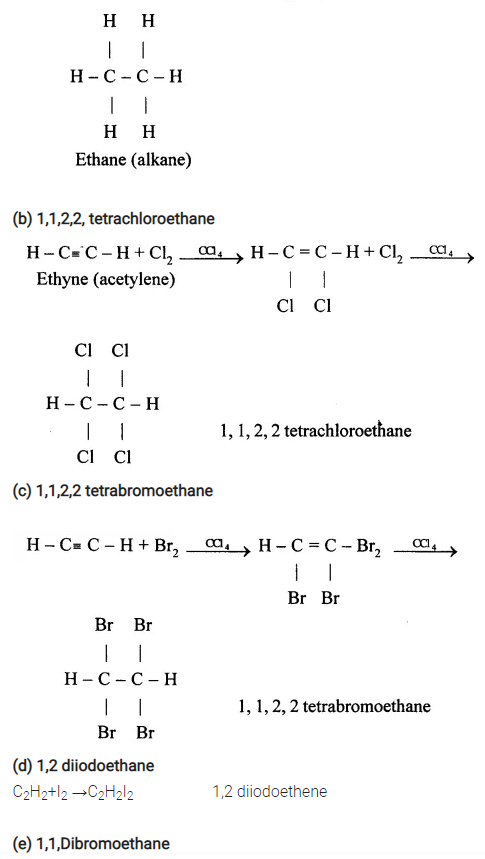
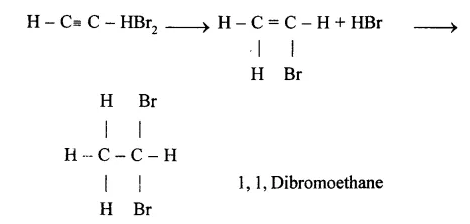
(f) Copper acetylide / Silver acetylide
HC ≡ CH+ 2CuCl + 2NH2OH →
Cu – C ≡ C – Cu + 2NH2Cl + 2H2O Copper acetylide
HC ≡ CH + 2AgNO3 + 2NH4OH →
Ag – C= C – Ag + 2NH2NO4 + 2H2O Silver acetylide
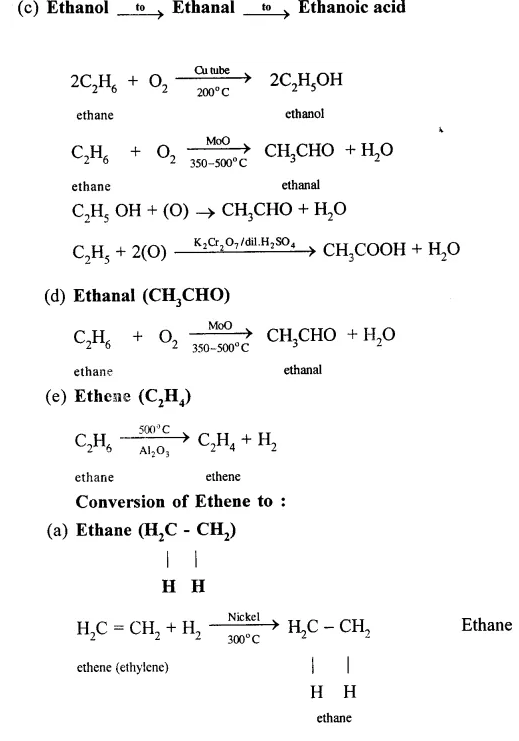
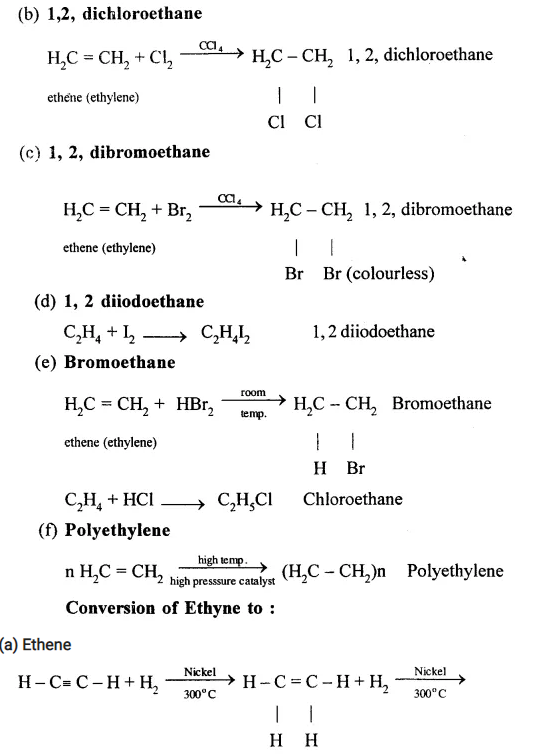
Conversion of Ethanol to :
(a) Carbon dioxide
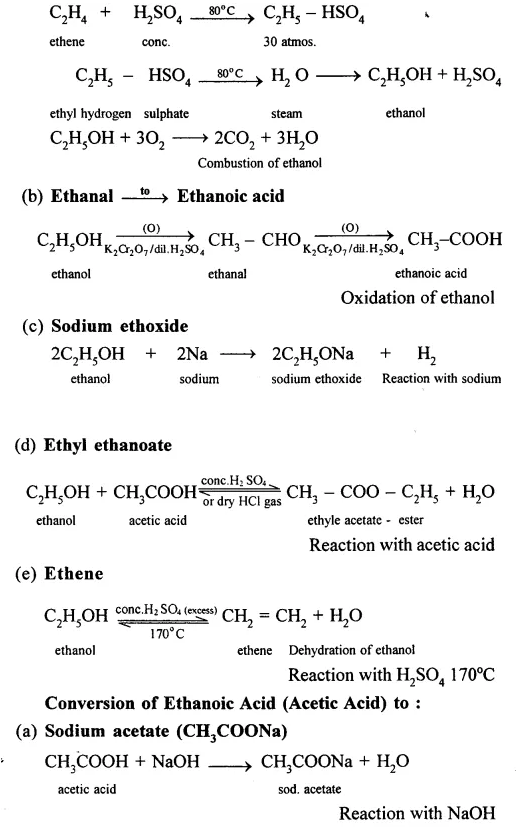
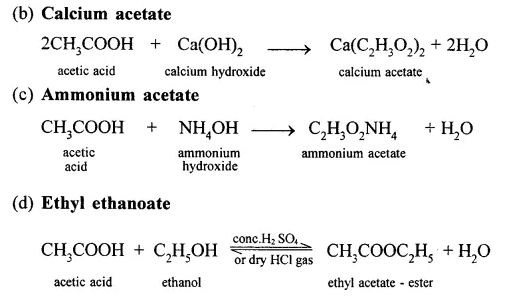
Question 16.
Give reasons for
- alkanes are said to be saturated organic compounds
- alkenes are known as olefins
- alkenes are more reactive than alkanes
- ethanoic acid is known as an aliphatic monocarboxylic acid.
Answer:
- Alkanes do not undergo addition reactions and that is why they are called saturated hydrocarbons or saturated organic compounds. In alkanes all the four valencies of carbon atom are fully satisfied by forming single covalent bonds.
- Alkenes are called olefins because alkenes on treatment with halogens form oily products. (Latin: oleum = oil, ficare = to make)
- Due to the presence of C = C (carbon – carbon double bond) alkenes are more reactive than alkanes.
- Ethanoic acid (CH3 – COOH) contains only one – COOH group (carboxylic acid group) that is why it is called a monocarboxylic acid. As ethanoic acid does not contain a benzene right it is an alphatic monocarboxylic acid.
Question 17.
Explain the terms –
- Denaturated alcohol
- Glacial acetic acid
- Esterification
Answer:
Denaturated alcohol –
Ethyl alcohol containing pyridine or copper sulphate is termed – denaturated alcohol. It is used for – industrial applications only and hence made undrinkable.
Glacial acetic acid –
Anhydrous acetic acid on cooling below 16.5°C crystallizes out in the pure form, forming a crystalline mass resembling ice. Hence pure acetic acid is called glacial acetic acid.
Esterification –
It is known as condensation of an alcohol with an acid. Acetic acid on heating with an alcohol and dehydrating agent [cone. H2SO2] gives an ester – ethyl acetate.
Question 18.
Give a chemical test for to distinguish between
- Ethane, ethene and ethyne
- Ethanol and ethanoic acid.
Answer:
- Tests to distinguish between ethane, ethene and ethyne
- Test — Br2 water test: Pass the gas through Br2 water,
Ethane : Brown colour of Br2 water is not discharged.
Ethene : Brown colour of Br2 water is discharged,
Ethyne : Brown colour of Br2 water is discharged. - Test — Baeyer’s reagent : Pass the gas through
Baeyer’s reagent (alkaline solution of KMnO4).
Ethane : Purple colour of Baeyer’s reagent is not discharged.
Ethene : Purple colour of Baeyers reagent is discharged.
Ethyne : Purple colour of Baeyers reagent is discharged. - Test : Pass the gas through ammoniacal cuprous chloride solution.
Ethane : No ppt.
Ethene : No ppt.
Ethyne : Red ppt. of copper acetylide is formed. - Test : Pass the gas through ammonical silver nitrate solution.
Ethane : No ppt.
Ethene : No ppt.
Ethyne : White ppt. of silver acetylide is formed.
- Test — Br2 water test: Pass the gas through Br2 water,
- Tests to distinguish between ethanol and ethanoic acid
- Test: Add a few drops of blue litmus solution to the given liquid.
Ethanol: No change in colour.
Ethanoic acid : Blue litmus turns red. - Test: Add a pinch of sodium carbonate to the given liquid. Ethanol: No action.
Ethanoic acid : Brisk effervescence with the evolution of C02.
- Test: Add a few drops of blue litmus solution to the given liquid.
Question 19.
Give the main uses of –
- Methane
- Ethane
- Ethene
- Ethyne
- Ethanol
- Ethanoic acid.
Answer:
The main uses of:
(1) Methane and
(2) Ethane –
(a) Illuminant and domestic fuel: In the form of natural gas or gobar gas. [Hydrocarbons – have high calorific value. They are easily combustible and the reaction is exothermic – releasing heat energy. Hence they are excellent fuels]
(b) In manufacture of chemicals : Used as :
- Chloroform : Solvent for rubber, waxes. As an anaesthesia.
- Carbon black : A black pigment in shoe polishes, printers ink etc.
- Formaldehyde : An antiseptic, preservative for biological specimens.
- Methanol : Solvent for varnishes, anti-freeze for automobiles.
- Ethanol: Solvent for resins, a low freezing liquid in thermometers.
(3) Use of Ethene –
(a) Production of oxy-ethylene torch : For welding purposes and cutting metals.
(b) Ripening of green fruits : Artificial ripening and preservation of fruits.
(c) Catalytic hydrogenation: Used in hardening of oils.
(d) It is also used in manufacturing of :
- Synthetic chemicals : Ethylene glycol [anti-freeze], di-ethyl ether [solvent], ethylene oxide [fumigant], mustard gas [chemical warfare],
- Polymers : Polyetheylene, polyvinyl chloride [P.V.C.]- used in packaging, insulators, containers, rain coats etc.
(4)Use of Ethyne –
(a) It is used for producing oxy-acetylene flame for welding and cutting purposes as it produces temperature as high as 3500°C.
(b) It is used as an illuminant in oxyacetylene lamp.
(c) It is used in the manufacture of solvent like westron (C2H2C14) and westrosol (CHCl = CC2).
(5) Ethanol – Main Uses of Ethanol
(a) As a solvent – For gums and resins
(b) In thermometers and spirit levels – Low freezing mobile liquid, [freezing point – 114.1°C].
(c) In manufacture of chemicals – Acetaldehyde [dyes], acetic acid [manufacture of vinegar], chloroform [antiseptic] diethyl ether [anaeshetic].
(6) Use Ethanoic acid –
(a) It is used as a solvent for many organic reactions.
(b) It is used as vinegar for preparing pickles etc.
(c) It is used for preparing various organic compounds like acetone, acetic anhydride ester etc.
(d) It is used as cogulating agent in rubber industries.
(e) It is used for making perfumes and medicines.
–Try Also :–


