Preparation of Hydrogen Class-8th Goyal Brothers ICSE Chemistry Solutions Ch-7 Unit-1 . We Provide Step by Step Answers of Objectives, True and False, Incorrect and Correct , Definitions , Match the followings and Short/Long Question Type answers of Ch-7 (Hydrogen) Unit-1. Visit official Website CISCE for detail information about ICSE Board Class-8
Preparation of Hydrogen Class-8th Goyal Brothers ICSE Chemistry Solutions Ch-7 Unit-1
| Board | ICSE |
| Class | 8th |
| Subject | Chemistry |
| Book Name | Goyal Brothers |
| Chapter-7 | Hydrogen |
| Unit-2 |
Preparation of Hydrogen |
| Topic | Solution of exercise questions |
| Session | 2023-24 |
OBJECTIVE TYPE QUESTIONS
Preparation of Hydrogen Class-8th Goyal Brothers ICSE Chemistry Solutions Ch-7 Unit-1
Que: A. Fill in the blank spaces by choosing the correct words from the given list :
List : nine, free hydrogen, cells, Henry Cavendish, magnesium oxide
1. Hydrogen gas was discovered by Henry Cavendish in 1766.
2. The sun has free hydrogen as largest constituent.
3. Every nine parts of water by weight contain one part of hydrogen by weight.
4. Hydrogen is vital constituent of cells of plants and animals.
5. When steam is passed over heated magnesium power, it forms magnesium oxide and hydrogen gas.
Que: B. Statements given below are incorrect. Write the correct statements :
Question: 1. Sodium metal reacts with hot water to form sodium hydroxide and hydrogen gas.
Answer: Sodium metal reacts with cold water to form sodium hydroxide and hydrogen gas.
Question: 2. Zinc dissolves in sodium hydroxide to form zinc hydroxide and hydrogen gas.
Answer: Zinc dissolves in sodium hydroxide to form sodium zincate and hydrogen gas.
Question: 3. In laboratory preparation of hydrogen gas, the reactants are aluminum and dilute sulphuric acid.
Answer: In laboratory preparation of hydrogen gas, the reactants are zinc and dilute sulphuric acid.
Question: 4. In laboratory preparation of hydrogen gas ferrous sulphate is used as catalyst.
Answer: In laboratory preparation of hydrogen gas copper sulphate is used as catalyst.
Question: 5. The acid used to make acidulated water in the electrolysis of water is carbonic acid.
Answer: The acid used to make acidulated water in the electrolysis of water is sulphuric acid.
Que: C. Match the statements in Column A, with those in Column B :
| Column A | Column B |
| 1. A metal which floats on the surface of cold water and reacts with it to form hydrogen gas. | (a) Zinc |
| 2. A metal which sinks in cold water and reacts with it to form hydrogen gas. | (b) Aluminum |
| 3. A silvery white metal in the form of a ribbon and reacts with steam on heating to form hydrogen. | (c) Sodium |
| 4. A metal which is used as reactant in the laboratory preparation of hydrogen gas. | (d) Magnesium |
| 5. A metal which reacts with sodium hydroxide to liberate hydrogen gas. |
(e) Calcium |
Answer:
| Column A | Column B |
| 1. A metal which floats on the surface of cold water and reacts with it to form hydrogen gas. | (a) Sodium |
| 2. A metal which sinks in cold water and reacts with it to form hydrogen gas. | (b) Calcium |
| 3. A silvery white metal in the form of a ribbon and reacts with steam on heating to form hydrogen. | (c) Aluminum |
| 4. A metal which is used as reactant in the laboratory preparation of hydrogen gas. | (d) Zinc |
| 5. A metal which reacts with sodium hydroxide to liberate hydrogen gas. |
(e)Magnesium |
Que: D. Write ‘True’ or ‘False’ for the following statements :
| Statements | True/False |
| 1. Magnesium reacts with caustic soda solution to liberate hydrogen gas. | F |
| 2. Sodium reacts with hydrochloric acid explosively. | T |
| 3. On electrolysis of acidulated water, two volumes of oxygen and one volume of hydrogen are formed. | F |
| 4. Zinc sulphate is used as catalyst during laboratory preparation of hydrogen gas. | F |
| 5. Calcium reacts with cold water and forms calcium hydroxide and hydrogen gas. |
T |
Que: E. Tick (√) the most appropriate answer:
1. The credit of discovery of hydrogen goes to :
(a) Lavoisier
(b) Robert Boyle
(c) Henry Cavendish
(d) Francis Bacon
Answer: option (c) Henry Cavendish is correct.
2. In free state hydrogen gas is present in :
(a) natural gas
(b) stars
(c) petroleum gas
(d) coal gas
Answer: option (b) stars is correct.
3. The metal which does not react with dilute sulphuric acid is :
(a) aluminum
(b) zinc
(c) copper
(d) iron
Answer: option (c) copper is correct.
4. The substance(s) which contain hydrogen in combined state is :
(a) carbohydrates
(b) fats
(c) proteins
(d) all of these
Answer: option (d) all of these is correct.
5. Red hot iron reacts with steam to form :
(a) ferrous oxide and hydrogen
(b) ferric oxide and hydrogen
(c) magnetic oxide of iron and hydrogen
(d) magnetic oxide of iron and oxygen
Answer: option (b) ferric oxide and hydrogen is correct.
STUDY QUESTIONS
Preparation of Hydrogen Class-8th Goyal Brothers ICSE Chemistry Solutions Ch-7 Unit-1
Question: 1. Name three metals which react with cold water to form hydrogen gas. Support your answer with fully balanced chemical equations.
Answer: reacts readily with water to form hydrogen gas & corresponding hydroxide.
Question: 2. Name three metals which in red hot state react with steam to liberate hydrogen gas, support your answer with fully balanced chemical equations.
Answer: Aluminum(Al), Zinc(Zn), and Iron(Fe) are the three metals that react with steam to form hydrogen gas.
2Al+3H2O→Al2O3+3H2
Zn+H2O→ZnO+H2
2Fe+3H2O→Fe2O3+3H2
Question: 3. Name four metals which react with caustic soda or caustic potash solution on boiling and liberate hydrogen gas. Support your answer by writing a fully balanced equation for any one of the above named metals.
Answer: The metals that react with caustic soda or caustic potash to liberate hydrogen gas are Al, Zn, Pb and Cd.
2Al(s) + 2NaOH(aq) + 6H2O(l) → 2Na[Al(OH)4] (sodium aluminate) + 3H2(g)
Question: 4. Name two metals which react with dilute sulphuric acid or dilute hydrochloric acid to liberate hydrogen gas. Support your answer by writing fully balanced equations of any one of the named metal with (a) dilute sulphuric acid (b) dilute hydrochloric acid.
Answer: Zn and Mg are two of the metals that can release hydrogen gas from dilute sulphuric and hydrochloric acid.
Zn + 2HCl → ZnCl2 + H2Mg + H2SO4 → MgSO4 + H2
Mg + 2HCl → MgCl2 + H2
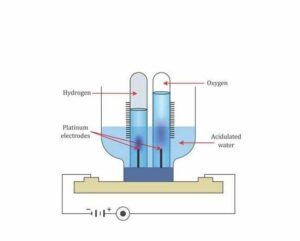
Question: 5. Briefly describe an experiment to prove that water contains two volumes of hydrogen and one volume of oxygen.
Answer:
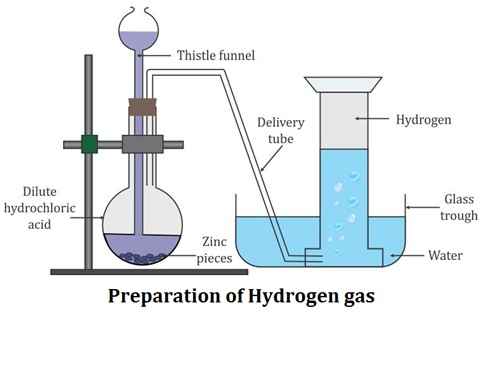
Question: 6. (a) By drawing neat diagram explain how hydrogen gas is collected in laboratory.
Answer:
a) 
Hydrogen gas is collected by the downward displacement of water.
Here, zinc reacts with hydrochloric acid and it will lead to the release of hydrogen gas and zinc chloride is formed.
(b) What is importance of copper sulphate solution in above reaction?
Answer: Copper is used in this reaction because it will act as a catalyst and will increase the rate of reaction.
— : end of Preparation of Hydrogen Class-8th Goyal Brothers ICSE Chemistry Solutions Ch-7 Unit-1 :–
Return to- ICSE Class -8 Goyal Brothers Chemistry Solutions
Thanks
Please share with yours friends if you find it helpful.



Please CHECK The QUESTION ANSWER AND EXCERCISE OF CH-7 HYDROGEN UNIT 2 YOU HAVE UPLOADED SAME solution of unit 1.
Please correct it as soon as possible
Sorry for inconvenience
now problems has been solved
visit again and see the modification