Sample Chemistry Paper ICSE Class-10 Specimen Model 2020. Specimen Chemistry ICSE Class-10 Paper for 2020. Sample Paper for ICSE Board Class-10 Chemistry. Hence by better practice of Sample Paper ICSE Chemistry 2020 is very helpful for ICSE student appearing in 2020 exam of council .
Sample Chemistry Paper ICSE Class-10 Specimen Model 2020
CHEMISTRY
Answers to this Paper must be written on the paper provided separately.
You will not be allowed to write during the first 15 minutes.
This time is to be spent in reading the Question Paper.
The time given at the head of this paper is the time allowed for writing the answers.
Section I is compulsory. Attempt any four questions from Section II.
The intended marks for questions or parts of questions are given in brackets [ ].
SECTION I (40 Marks)
Attempt all questions from this Section
Question 1
(a) Choose the most appropriate answer.
(i)
Which of the following is a common characteristic of a covalent compound?
A High melting point.
B Conducts electricity when it is in the molten state.
C Consists of molecules.
D Always soluble in water.
(ii)
Ammonium hydroxide will produce a reddish brown precipitate when added to
a solution of :
A CuSO4
B Zn(NO3)2
C FeSO4
D FeCl3
(iii)
A salt which in solution gives a bluish white precipitate with NaOH solution
and a white precipitate with BaCl2 solution
A CuSO4
B FeSO4
C Fe2(SO4)3
D CuCl2
(iv)
The gas law which relates the volume of a gas to moles of the gas is:
A Avogadro’s Law
B Gay-Lussac’s Law
C Boyle’s Law
D Charle’s Law
(v)
During the electrolysis of acidified water which of the following takes place:
A Oxygen is released at cathode.
B Oxygen is released at anode.
C Hydrogen is released at anode.
D Sulphur dioxide is released at anode.
(vi)
Duralumin is an alloy of
A Al and Cu
B Cu and Sn
C Al and Ag
D Al and Fe
(vii)
Hydrogen chloride can be obtained by adding concentrated Sulphuric acid to:
A NaCl
B Na2SO4
C Na2CO3
D NaNO3
(viii)
Which of the following reactions gives copper as a product
A Passing dry ammonia over heated copper oxide.
B Adding dilute hydrochloric acid to copper oxide.
C Heating copper oxide.
D Passing oxygen over heated copper oxide?
(ix)
Formation of chloroform from methane and chlorine is an example of:
A Addition
B Dehydration
C Substitution
D Elimination
(x)
The element with the highest ionization potential in the periodic table is:
A He
B Ne
C Ar
D Xe
(b)
The equation for the action of heat on calcium nitrate is:
2Ca(NO3)2 → 2CaO + 4 NO2 + O2
(i) How many moles of NO2 are produced when 1 mole of Ca(NO3)2 decomposes?
(ii) What volume of O2 at S.T.P. will be produced on heating 65.6 g of Ca (NO3)2?
(iii) Find out the mass of CaO formed when 65.6 g of Ca(NO3)2 is heated.
(iv) Find out the mass of Ca(NO3)2, required to produce 5 moles of gaseous
products.
(v) Find out the mass of Ca(NO3)2 required to produce 44. 8 L of NO2 at S.T.P.
(Relative molecular mass of Ca(NO3)2 = 164 and of CaO = 56) [5]
(c)
Name the organic compound prepared by each of the following reactions:
(i)CH3 COONa + NaOH →CaO
(ii) CaC2 + H2O →
(iii) C2H5Br + KOH(alc)→
(iv) C2H5Br + KOH (aq)→
(v)C2H5OH + CH3COOH →
(d)
Identify the following substances:
(i) An acidic gas which gives dense white fumes with NH3
(ii) An alkane which can also be called a green house gas.
(iii) A solid which when kept in the open, forms a solution after sometime.
(iv) An alloy used in electrical fittings.
(v) A metal which gives hydrogen gas on reacting with both dilute acid and alkali.
(e)
Write equations for the following reactions:
(i) Aluminium oxide and Sodium hydroxide.
(ii) Zinc and dilute sulphuric acid.
(iii) Nitrogen dioxide and water.
(iv) Concentrated sulphuric acid and sugar.
(v) Copper with concentrated nitric acid. [5]
(f)
Name the following:
(i) Second member of alkene series
(ii) First member of alkane series
(iii) Third member of aldehyde series.
(iv) Second member of carboxylic acid.
(v) Fourth member of alcohol series. [5]
(g)
Write the I.U.P.A.C. names of the following compounds:
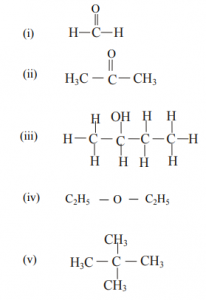
SECTION II (40 Marks)
Attempt any four questions from this Section.
Question 2.
(a)
The following questions refer to the periodic table:
(i) Name the second last element of the period 3.
(ii) How many elements are in the second period?
(iii) Name the element which has the highest electron affinity.
(iv) Name the element which has the highest electro negativity.
(v) Name the element which may be placed on group 1 but is not a metal. [5]
(b)
Fill in the blanks using the correct options:
(i) Metals have —— ionisation potential. (low/ high)
(ii) Group 18 elements have —— valence electrons (4 / 8) with the
exception of —- ( He / Ne) with ——– electrons (2 / 8) in valence
shell.
(iii) Group 2 elements are called —– metals (alkali / alkaline earth). [5]
Question 3.
(a)
Draw different isomers having the following molecular formula:
(i) C5H12 (chain)
(ii) C4H8 (position). [5]
(b)
What is denatured alcohol? [1]
(c)
Give two important uses of ethanol. [2]
(d)
Write equations for:
(i) Preparation of ethanol by hydration of
C2H4
(ii) Preparation of acetic acid from ethanol. [2]
Question. 4
(a)
Name the method by which following compounds can be prepared:
Select the appropriate method from the following list Neutralization; direct
combination; precipitation; metal + acid – use a method only once.
(i) Sodium sulphate
(ii) Silver chloride
(iii) Iron sulphide. [3]
(b)
How will you distinguish between following pairs of compounds using NH4OH
(i) Copper sulphate and iron(II) sulphate.
(ii) Zinc nitrate and lead nitrate.
(iii) Iron(II) sulphate and iron(III) sulphate. [3]
(c)
Name:
(i) A greenish yellow gas with pungent smell.
(ii) An oxide which is yellow when hot and white when cold.
(iii) A chemical used to deplete ozone layer.
(iv) A crystalline salt without water of crystallization. [4]
Question. 5
(a)
Name one:
(i) metal liquid at room temperature.
(ii) non-metal which is a conductor of electricity.
(iii) neutral oxide.
(iv) metallic oxide which cannot be reduced by hydrogen.
(v) non-metal which has lustre. [5]
(b)
(i) Name the chief ore of aluminium.
(ii) Name the process used to concentrate the above mentioned ore.
(iii) Why is alumina added to cryolite in the electrolytic reduction of
aluminium?
(iv) Give cathode and anode reactions involved in extraction of aluminium
from its above mentioned ore.
(v) Name the process used for the concentration of zinc blende. [5]
Question 6.
(a) Draw a neat and well labelled diagram for the silver plating on an iron
spoon. [3]
(b) Copy and complete the following table related to electrolysis.

(c) Classify the following as oxidation and reduction reaction, also complete the
reaction.
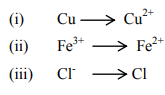
Question. 7
(a) A compound has the following percentage composition by mass:
Carbon – 54.55%, Hydrogen – 9.09% and Oxygen – 36.26%. Its vapour density
is 44. Find the Empirical and Molecular formula of the compound.
(H = 1; C = 12; O = 16) [5]
(b)
Give the electron dot structure of the following:
(i)NH3
(ii)CH4
(iii) H3O+
(c)
Compare the properties of covalent and electrovalent compounds on the
following points:
(i) Solubility
(ii) Structure.
–: Try Also:–
- Mathematics Sample Paper for ICSE Class-10 Specimen
- Physics Sample Paper for ICSE Class-10 Specimen
- History and Civics Sample Paper for ICSE Class-10 Specimen
- Biology Sample Paper for ICSE Class-10 Specimen
- Chemistery Sample Paper for ICSE Class-10 Specimen ( Currently Open )
- Geography Sample Paper for ICSE Class-10 Specimen
- Hindi Sample Paper for ICSE Class-10 Specimen
- English Literature Sample Paper for ICSE Class-10 Specimen
- English Language Sample Paper for ICSE Class-10 Specimen
Previous Year Solved Question Paper for ICSE Board



ans to this?