Second Law of Thermodynamics Numerical on Heat Engine Class-11 Nootan ISC Physics Solutions Ch-21. Step by step solutions of Kumar and Mittal Physics of Nageen Prakashan as council latest prescribe guideline for upcoming exam. Visit official Website CISCE for detail information about ISC Board Class-11 Physics.
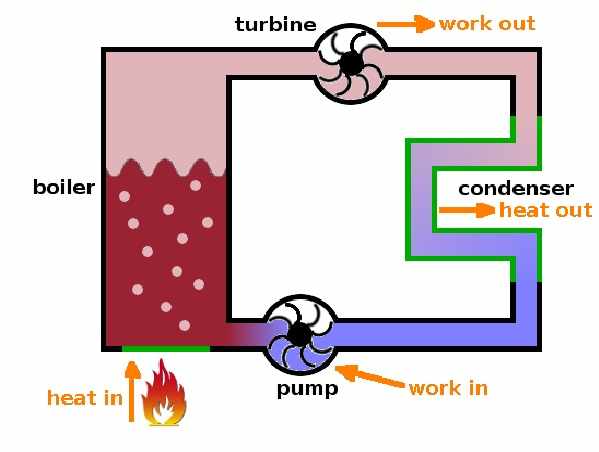
Second Law of Thermodynamics Numerical on Heat Engine
(Class-11 Nootan ISC Physics Solutions Ch-21)
| Board | ISC |
| Class | 11 |
| Subject | Physics |
| Book | Nootan |
| Chapter-21 | Heat Engine: Second Law of Thermodynamics |
| Topics | Numerical on Heat Engine |
| Academic Session | 2024-2025 |
Second Law of Thermodynamics Numerical on Heat Engine
(Class-11 Nootan ISC Physics Solutions Ch-21)
Definition: second law states that it is impossible for a heat engine to convert all the heat it receives from a thermal energy reservoir to work
Que-1: A Carnot’s engine has the same efficiency (i) between 100 K and 500 K and (ii) between T K and 900 K. Calculate the temperature T of the sink.
Ans: η = 1 – T2/T1
=> 1 – 100/500 = 1 – T / 900
=> T = 900 x 100 / 500
=> 180 K
Que-2: Calculate the difference in efficiencies of a Carnot’s heat engine working between (i) 400 K and 350 K, (ii) 350 K and 300 K.
Ans: η1 = (1 – 350/400) x 100%
=> 100 – 87.5 = 12.5 %
η2 = (1 – 300/350) x 100%
=> 100 – 600/7 = 14.2857 %
Difference = 14.286 – 12.5 = 1.786 %
Que-3: The temperature of source of a Carnot’s heat engine is 127°C. It takes 500 calories of heat from the source and rejects 400 calories to the sink per cycle. Calculate the temperature of the sink and the efficiency of the engine.
Ans: Q1/Q2 = T1/T2
=> 500 / 400 = 273+127 / T2
=> T2 = 4 x 400 / 5 = 320 K
=> 320 – 273 = 47 °C
and efficiency = 1 – T2 / T1
=> 1 – 4/5 = 1/5
=> 1/5 x 100% = 20 %
Que-4: A Carnot’s engine absorbs 1000 J of heat from a source at temperature 127°C and rejects 600 J of heat to the sink during each cycle. Calculate the amount of useful work done during each cycle, efficiency of the engine and temperature of the sink.
Ans: W = Q1 – Q2
=> 1000 – 600 = 400 J
efficiency = 1 – Q2 / Q1
=> 1 – 600/1000 = 0.4
=> 0.4 x 100% = 40 %
again T2 / T1 = Q2 / Q1
=> T2 / 273 + 127 = 600 / 1000
=> T2 = 400 x 600 / 1000 = 240 K
=> 240 – 273 = -33 °C
Que-5: A Carnot’s engine works between two temperatures whose difference is 100 K. If it absorbs 746 J of heat from the source and gives 546 J to the sink, calculate the temperatures of the source and the sink.
Ans: T1 / T2 = Q1 / Q2
=> T2 + 100 / T2 = 746 / 546
=> 1 + 100 / T2 = 373 / 273
=> 100 / T2 = 373 / 273 – 1
=> 100 / T2 = 100 / 273
=> T2 = 273 K
and T1 = T2 + 100
=> 273 + 100 = 373 K
Que-6: A Carnot’s ideal heat engine operates between 227°C and 127°C. It absorbs 600 calories of heat from the source. How much work per cycle is the engine capable of performing? (1 calorie = 4.2 joule)
Ans-6 T2 / T1 = Q2 / Q1
=> 273 + 127 / 273 + 227 = Q2 / 600
=> 400 / 500 = Q2 / 600
=> Q2 = 480 Cal
W = Q1 -Q2
=> 600 – 480 = 120 Cal
=> 120 x 4.2 = 504 J
Que-7: A Carnot’s reversible engine works with an efficiency of 50%. During each cycle, it rejects 150 calories of heat at 30°C. Calculate (i) temperature of the source, (ii) work done by the engine per cycle. (1 Cal = 4.2 J)
Ans-7 1 – T2 / T1 = 50 / 100
=> T2 / T1 = 1/2
=> 273 + 30 / T1 = 1/2
=> T1 = 606 K
=> 606 – 273 = 333 °C
again Q2 / Q1 = T2 / T1
=> 150 / Q1 = 1/2
=> Q1 = 300 Cal
∴ W = Q1 – Q2
=> 300 – 150 = 150 Cal
=> 150 x 4.2 = 630 J
Que-8: The temperature of the sink of a Carnot engine is 27°C. If the efficiency of the engine is 40%, find the temperature of the source.
Ans: 1 – T2 / T1 = 40 / 100
=> T2 / T1 = 3/5
=> 273 + 27 / T1 = 3/5
=> T1 = 300 x 5 /3
=> T1 = 500 K
=> 500 – 273 = 227 °C
Que-9: A reversible engine converts one-sixth of heat absorbed at the source into work. When the temperature of the sink is reduced by 82°C, the efficiency is doubled. Find the temperatures of the source and the sink.
Ans: 1 – T2 / T1 = 1 / 6
=> T2 / T1 = 5 / 6
again 1 – (T2 – 82 / T1) = 2 / 6
=> T2 – 82 / T1 = 2 / 3
=> T1 = 3 (T2 – 82) / 2
=> 2 T2 / 3 (T2 – 82) = 5/6
=> 4 T2 = 5 T2 – 410
=> T2 = 410 K
=> 410 – 273 = 137 °C
again T2 / T1 = 5/6
=> 410 / T1 = 5 / 6
=> T1 = 492 K
=> 492 – 273 = 219 °C
Que-10: A Carnot engine whose low-temperature reservoir is at 7°C has an efficiency of 50%. It is desired to increase the efficiency to 70%. By how many degree should the temperature of the high-temperature reservoir be raised?
Ans: 1 – T2 / T1 = 50 / 100
=> T2 / T1 = 1/2
=> 273 + 7 / T1 = 1 / 2
=> T1 = 560 K
again 1 – T2 / T1′ = 70 / 100
=> T2 / T1′ = 3 / 10
=> 273 + 7 / T1′
=> T1′ = 280 x 10 / 3
=> T1′ = 2800 /3 = 933 K
∴ ΔT = 933 – 560 = 373 °C
— : end of Second Law of Thermodynamics Numerical on Heat Engine Class-11 ISC Physics Solutions :–
Return to : – Nootan Solutions for ISC Class-11 Physics
Thanks
Please Share with your friends if helpful


