Study of First Element Hydrogen Exe-6B Chemistry Class-9 ICSE Selina Publishers Solutions Chapter-6. Step By Step ICSE Selina Concise Solutions of Chapter-6 Study of First Element Hydrogen with All Exercise including MCQs, Very Short Answer Type, Short Answer Type, Long Answer Type, Numerical and Structured/Application Questions Solved . Visit official Website CISCE for detail information about ICSE Board Class-9.
Study of First Element Hydrogen Exe-6B Chemistry Class-9 ICSE Concise Selina Publishers
| Board | ICSE |
| Publications | Selina Publication |
| Subject | Chemistry |
| Class | 9th |
| Chapter-6 | Study of First Element Hydrogen |
| Book Name | Concise |
| Topics | Solution of Exercise – 6B |
| Academic Session | 2023-2024 |
B. Exercise – 6B
Study of First Element Hydrogen Class-9 Chemistry Concise Solutions
Page-111
Question 1.
Hydrogen can be prepared with the metal zinc by using:
(i) acid
(ii) alkali
(iii) water
Give an equation in each case.
Answer:
(i) Zn + HCl → ZnCl2 + H2
(ii) Zn + 2NaOH → Na2ZnO2 + H2
(iii) Zn + H2O → ZnO + H2
Question 2.
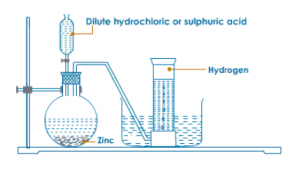
For laboratory preparation of hydrogen, give the following:
(a) materials used
(b) method of collection
(c) chemical equation
(d) fully-labelled diagram
Answer:
(a) Granulated zinc, dilute HCl or dil. H2SO4
(b) It is collected by the downward displacement of water.
(c) Zn + HCl → ZnCl2 + H2
(d)
Question 3.
(a) Name the impurities present in hydrogen prepared in the laboratory.
(b) How can these impurities be removed?
Answer:
(a) Hydrogen sulphide, sulphur dioxide, oxides of nitrogen, phosphine, arsine, carbon dioxide and watervapour are impurities present in the laboratory.
(b) The impurities can be removed from hydrogen by passing it through
Silver nitrate solution to remove arsine and phosphine.
AsH3 + 6AgNO3 → Ag3As + 3AgNO3 + 3HNO3
PH3 + 6AgNO3 → Ag3P + 3AgNO3 + 3HNO3
Lead nitrate solution to remove hydrogen sulphide.
Pb(NO3)2 + H2S → PbS + 2HNO3
Caustic potash solution to remove sulphur dioxide, carbon dioxide and oxides of nitrogen.
SO2 + 2KOH → K2SO3 + H2O
CO2 + 2KOH→ K2CO3+ H2O
2NO2 + 2KOH →KNO2 + KNO3 + H2O
A drying agent used to dry the gas. Common drying agents such as fused calcium chloride, caustic potash stick and phosphorus pentoxide remove water vapour.
So, the gas is purified and dried and then collected over mercury because mercury does not react with it.
Question 4.
Which test should be made before collecting hydrogen in a gas jar?
Answer:
Test: Collect some amount of gas in a test tube and take it to a flame.
If the gas burns quietly, then there is no more air in the flask.
Question 5.
Why nitric acid is not used in the preparation of hydrogen?
Answer:
Nitric acid is a powerful oxidising agent, and the oxygen formed due to its decomposition oxidiseshydrogen to give water thus defeating the purpose of the reaction.
3Zn + 8HNO3 → 3Zn(NO3)2 + 4H2O + 2NO
Question 6.
Why hot concentrated sulphuric acid is not used in the preparation of hydrogen?
Answer:
Conc. sulphuric acid is not used in the preparation of hydrogen as it will produce sulphur dioxide.
Zn + 2H2SO4 →ZnSO4 + SO2 + 2H2O
Question 7.
Hydrogen is manufactured by ‘Bosch Process’.
(a) Give the equations with conditions.
(b) How can you obtain hydrogen from a mixture of hydrogen and carbon monoxide?
Answer:
(a) C + H2O→ (CO + H2) – ∆
(b) (CO + H2)+ H2O→CO2 + 2H2 + ∆
The mixture is passed through ammoniacal cuprous chloride solution in order to dissolve any uncombined carbon monoxide.
CuCl + CO + 2H2O →CuCl.CO.2H2O
Question 8.
Give equations to express the reaction between:
(a) Steam and red hot iron
(b) Calcium and water
Answer:
(a) 3Fe + 4H2O⇋ Fe3O4 + 4H2
(b) Ca + 2H2O ⇋ Ca(OH)2 + H2
Question 9.
A small piece of calcium metal is put into a small trough containing water. There is effervescence and white turbidity is formed.
(a) Name the gas formed in the reaction. How would you test the gas?
(b) Write an equation for the reaction.
(c) What do you observe when a few drops of red litmus solution are added to the turbid solution.
Answer:
Hydrogen gas. When red litmus is introduced in the solution, it turns blue.
(a) Ca + 2H2O → Ca(OH)2 + H2
(b) The solution turns blue.
(c) If dilute hydrochloric acid is added to the turbid solution, then they react and neutralise each other, forming the soluble salt calcium chloride (CaCl2) and water.
Ca(OH)2 + 2HCl → CaCl2 + 2H2O
Question 10.
Thin strips of magnesium,copper and iron are taken.
(a) Write down what happens when these metals are treated as follows:
(i) Heated in presence of air
(ii) Heated with dil.HCl
(iii)Added to an aqueous solution of zinc sulphat
(b) Arrange these metals in descending order of reactivity.
Answer:
(a)
(i) On heating thin strips of magnesium, copper and iron, they form oxides.
(ii) Magnesium and iron react with HCl liberating hydrogen and forming their respective salts. Hydrogen cannot be prepared from metals which are below it in the activity series of metals (such as copper) because only metals which are more reactive than hydrogen can displace it from acids.
(iii) Only magnesium will displace zinc from zinc sulphate solution because magnesium is more reactive than zinc in the activity series of metals. No reaction takes place in case of copper and iron because they are less reactive than zinc.
(b)Mg > Fe > Cu
B. Exercise – 6B
Study of First Element Hydrogen Class-9 Chemistry Concise Solutions
Page-112
Question 11.
Choose the correct option:
(a )Hydrogen is evolved by the action of cold dil. HNO3 on
A. Fe B. Cu C. Mg D. Zn
(b) Which metal absorbs hydrogen?
A. Al B. Fe C. Pd D. K
(c)The composition of nucleus of deuterium is
A. 1 e– and 1P B. 1 P and 1 A
C. 1 n and 1 e– D. 2P and 1 e–
(d) Elements which show unique nature in the preparation of hydrogen are:
A. Na, K, Li B. Mg, Ca, Ba
C. Al, Zn, Pb D. Fe, Cu, Ag
Answer:
(a) C. Mg
(b) C. Pd
(c) C. 1 n and 1 e–
(d) C. Al, Zn, Pb
Question 12.
Give reasons for the following:
(a) Zinc granules are used in lab preparation of hydrogen.
(b) Purified and dried hydrogen is collected over mercury.
(c)The end of the thistle funnel should be dipped under acid
(d) Dilute sulphuric acid cannot be replaced by concentrated acid in the preparation of hydrogen.
Answer:
(a) Zinc granules are preferred over pure zinc in the lab preparation of hydrogen because the impurity present in granulated zinc is copper, whose catalysing effect speeds up the rate of the reaction.
(b) Purified and dried hydrogen is collected over mercury because mercury has no reaction with it.
(c) The end of the thistle funnel should be dipped under acid so as to prevent the gas from escaping from the thistle funnel.
(d) Dilute sulphuric acid cannot be replaced by concentrated acid in the preparation of hydrogen because it is a strong oxidising agent and it will produce sulphur dioxide.
— : End of Study of First Element Hydrogen Exe-6B Answer Class-9 ICSE Chemistry Solutions :–
Return to Return to Concise Selina ICSE Chemistry Class-9
Thanks
Please share with your friends


