Thermodynamics Numerical on First Law Class-11 Nootan Physics Solutions Ch-19. Step by step solutions of Kumar and Mittal Physics of Nageen Prakashan as council latest prescribe guideline for upcoming exam. Visit official Website CISCE for detail information about ISC Board Class-11 Physics.
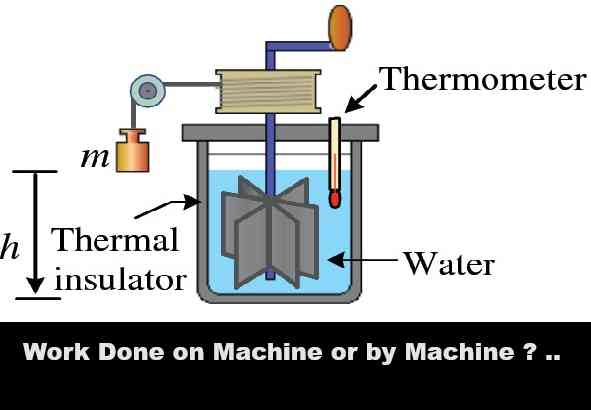
Thermodynamics Numerical on First Law Class-11 Nootan Physics Solutions
| Board | ISC |
| Class | 11 |
| Subject | Physics |
| Book | Nootan |
| Chapter-19 | Thermodynamics |
| Topics | Thermodynamics Numerical on First Law |
| Academic Session | 2024-2025 |
Numerical on First Law of Thermodynamics
( Class-11 Nootan Physics Solutions of Ch-19 Questions )
Que-19: The volume of a gas at the atmospheric pressure is 2.0 litre. On giving 300 joule of heat to the gas, its volume increases to 2.5 litre at the same pressure. Determine the change in the internal energy of the gas. Atmospheric pressure = 1.0 × 10^5 N m^-2 , 1 litre = 10^-3 m³.
Ans: ΔU = ΔQ – ΔW {ΔW = P . ΔV}
=> ΔU = 300 – (25.2) x 10^-3 x 10^5
=> 300 – 50 = 250 J
Que-20: How much external work would have to be done in reducing the volume of an ideal gas by 2.4 × 10^-4 m³ at normal temperature and normal (constant) pressure (1.0 x 10^5 N m^-2)? If, on absorbing 12 J of energy, the temperature of the gas rises through 1°C, calculate the final temperature of the compressed gas.
Ans: ΔW = P . ΔV
=> 1 x 10^5 x 2.4 x 10^4 = 24 J
∴ rise in temp
=> 24 / 12 = 2 °C
Que-21: 1671 cm³ of water vapour is formed from 1 cm³ of water at atmospheric pressure (1.01 × 10^5 N m^-2) and 100°C. Latent heat of vaporisation is 540 Cal g^-1. How much increase in the internal energy will take place when one gram of water is converted into vapour at atmospheric pressure? (J = 4.2 J Cal^-1).
Ans: ΔU = ΔQ – ΔW
ΔU = 540 – (10^5 x (167 – 1) x 10^-6 / 4.2)
=> 540 – 40 = 500 Cal
Que-22: The volume of 1.0 kg of water at 100°C is 1 × 10^-3 m³ and the volume of 1.0 kg of steam at normal pressure is 1.671 m³. The latent heat of steam is 2.3 × 10^6 J kg^-1 and the normal pressure is 1.0 × 10^5N m^-2. How much work will be done in converting 5.0 kg of water at 100°C into steam at the same temperature and normal pressure? What will be the increase in the internal energy of water in this process?
Ans: ΔW = ΔQ – ΔU
=> ΔW = P . ΔV
=> 10^5 x 5 (1.671 – 0.001)
=> 8.35 x 10^5 J
again ΔU = ΔQ – ΔW
=> m L – ΔW
=> 5 x 2.3 x 10^6 – 8.35 x 10^5
=> 1.066 x 10^7 J
Que-23: 2000 calories of heat are given to a thermodynamic system and the system does 3350 joule of external work. In this process the internal energy of the system is increased by 5030 joule. Calculate the value of the conversion factor J.
Ans: ΔQ = ΔU + ΔW
=> 2000 = 5030 / J + 3350 / J
=> 2000 = 8380 / J
=> J = 8380 / 2000 = 4.19 J Cal^-1
Que-24: If, on giving heat amounting 40 J to a system, the work done is – 8 J, then what will be the change in the internal energy of the system?
Ans: ΔU = ΔQ – ΔW
=> ΔU = 40 – (-8)
=> 48 J
Que-25: Heat equivalent to 50 J is supplied to a thermodynamic system and 10 J work is done on the system. What is the change in the internal energy of the system in this process?
Ans: ΔU = ΔQ – ΔW
=> 50 – (-10) = 60 J
Work done on system is negative
Que-26: 1000 Cal heat was given to a system. 418 J work was done by the system and 100 Cal heat was destroyed. What was the change in the internal energy of the system?
Ans: Net heat given to system
=> 1000 – 100 = 900 Cal
=> 900 x 4.18 = 3762 J
again ΔU = ΔQ – ΔW
=> ΔU = 3762 – 418 = 3344 J
Que-27: A system is given 200 Cal of heat and also 210 J of work is done on it. If 50 Cal of heat is lost due to conduction, find the change in the internal energy of the system. (1 Cal = 4.2 J)
Ans: Net heat given to system
=> 200 – 50 = 150 Cal
=> 150 x 4.2 = 630 J
again, ΔU = ΔQ – ΔW
=> ΔU = 630 -(-230) = 840 J
Work done on system is negative
— : end of Thermodynamics Numerical on First Law Class-11 Nootan Physics Solutions :–
Return to : – Nootan Solutions for ISC Class-11 Physics
Thanks
Please Share with your friends if helpful