Water Exe-3B Chemistry Class-9 ICSE Selina Publishers Solutions Chapter-3. Step By Step ICSE Selina Concise Solutions of Chapter-3 Water with All Exercise including MCQs, Very Short Answer Type, Short Answer Type, Long Answer Type, Numerical and Structured/Application Questions Solved . Visit official Website CISCE for detail information about ICSE Board Class-9.
Water Exe-3B Chemistry Class-9 ICSE Concise Selina Publishers
| Board | ICSE |
| Publications | Selina Publication |
| Subject | Chemistry |
| Class | 9th |
| Chapter-3 | Water |
| Book Name | Concise |
| Topics | Solution of (Solution) Exercise – 3B |
| Academic Session | 2023-2024 |
B. Exercise – 3B
Water Class-9 Chemistry Concise Solutions
Page-49
Question 1.
Explain the terms:
(a) Solution
(b) Solute
(c) Solvent
Answer:
(a) Solution: A Solution is a homogeneous mixture of two or more substances, the components of which cannot be seen separately.
(b) Solute: A solute is the substance which dissolves in a solvent to form a Solution.
(c) Solvent: A solvent is the medium in which a solute dissolves.
Question 2.
Explain why a hot saturated Solution of potassium nitrate forms crystals as it cools.
Answer:
Solubility of nitrates decreases with a fall in temperature. Thus, when a hot saturated Solution of potassium nitrate cools, it forms crystals as it separates from the Solution.
Question 3.
Give three factors which affect the solubility of a solid solute in a solvent.
Answer:
Three factors on which the solubility of a solid depend:
(i) Temperature
(ii) Nature of the solid
(iii) Nature of the solvent
Question 4.
(a) If you are given some copper sulphate crystals, how would you proceed to prepare its saturated Solution at room temperature?
(b) How can you show that your Solution is really saturated?
Answer:
(a) Take 100 g of distilled water in a beaker. Add to this one gram of copper sulphate crystals.
(b) This mixture with the help of a glass rod and dissolve the copper sulphate crystals. Similarly, go on dissolving more copper sulphate (1 gram at a time) with constant and vigorous stirring. A stage is reached when no more copper sulphate dissolves. It is called a saturated Solution at this temperature.
Question 5
(a) Define:
(i) Henry’s law and (ii) Crystallisation (iii) Seeding.
(b) State the different methods of crystallisation.
Answer:
(a)
(i) Henry’s law:
At any given temperature, the mass of a gas dissolved in a fixed volume of a liquid or Solution is directly proportional to the pressure on the surface of a liquid.
(ii) Crystallisation:
It is the process by which crystals of a substance separate out on cooling its hot saturated Solution.
(iii) Seeding:
This process of inducting crystallisation by adding a crystal of pure substance into saturated solution is called seeding.
(b) In the laboratory, crystals may be obtained by the following methods:
(i) By cooling a hot saturated Solution gently
(ii) By cooling a fused mass
(iii) By sublimation
(iv) By slowly evaporating a saturated Solution
Question 6.
What would you observe when crystals of copper (II) sulphate and iron (II) sulphate are separately heated in two test tubes strongly?
Answer:
Action of heat on copper (II) sulphate crystals
When copper (II) sulphate crystals are heated in a hard glass test tube, the following observations are observed:

(i) The crystals are converted to a powdery substance.
(ii) The crystals lose their blue coloration on further heating.
(iii) Steaming vapors are produced inside the tube which condense near the mouth of the tube to form a colorless liquid.
(iv) On further heating, steam escapes from the mouth of the tube and water gets collected in a beaker placed under the mouth of the tube.
(v) On further heating, the residue changes to a white powder and steam stops coming out.
| CuSO4.5H2O → CuSO4 + 5H2O |
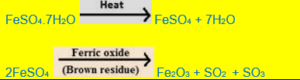
Action of heat on iron (II) sulphate
When iron (II) sulphate is heated in a test tube, the following is observed:
(i)The crystals crumble to a white powder and a large amount of steam and gas are given out.
(ii)On strong heating, a brown residue of ferric oxide (Fe2O3) is produced and a mixture of SO2 and SO3 is given off.
Question 7.
Give the names and formulae of two substances in each case:
(a) Hydrated substance
(b) Anhydrous substance
(c) Liquid drying agent
(d) A basic drying agent
Answer:
(a)
(i) Washing soda crystals: Na2CO3.10H2O
(ii) Blue vitriol: CuSO4.5 H2O
(b)
(i) Table salt: NaCl
(ii) Nitre: KNO3
(c)Sulphuric acid: H2SO4
(d)Quick lime: CaO
Question 8.
What is the effect of temperature on solubility of KNO3 and CaSO4 in water?
Answer:
Solubility of potassium nitrate (KNO3) in water increases with an increase in temperature.
Solubility of calcium sulphate (CaSO4) in water decreases with an increase in temperature.
Question 9.
Solubility of NaCl at 40oC is 36.5 g. What is meant by this statement?
Answer:
Solubility of NaCl at 40°C is 36.5 g means that 36.5 g of NaCl dissolves in 100 g of water at a temperature of 40°C.
Question 10.
Which test will you carry out to find out if a given Solution is saturated or unsaturated or supersaturated?
Answer:
A Solution in which more of a solute can be dissolved at a given temperature is an unsaturated Solution.
The Solution in which no more solute can be dissolved at a given temperature is a saturated Solution at that temperature.
Solution in which some solute separates on cooling slightly is a super saturated Solution.
Question 11.
What is the effect of pressure on solubility of gases? Explain with an example.
Answer:
With an increase in pressure, the solubility of a gas in water increases.
With an increase in temperature, the solubility of a gas in water decreases.
For example, the solubility of carbon dioxide in water under normal atmospheric pressure is low, but when the water surface is subjected to higher pressure, a lot more of CO2 gas gets dissolved in it.
Similarly, in case of soda water, on opening the bottle, the dissolved gas rapidly bubbles out because the pressure on the surface of the water suddenly decreases.
Question 12.
State the term:
(a) A Solution where solvent is a liquid other than water.
(b) When a substance absorbs moisture on exposure to moist air and dissolves in the absorbed water and turned to Solution.
(c) A substance which contains water of crystallisation.
(d) When a substance absorbs moisture from the atmosphere but does not form a Solution.
(e) When a compound loses its water of crystallisation on exposure to dry air.
(f) The substance that can remove hydrogen and oxygen atoms in the ratio of 2:1(in the form of water) from the compound.
Answer:
(a) Non-aqueous Solution
(b) Deliquescence
(c) Hydrated substance
(d) Hygroscope
(e) Efflorescence
(f) Dehydrating agent
Question 13.
Explain why:
(a) Water is an excellent liquid to use in cooling systems.
(b) A Solution is always clear and transparent.
(c) Lakes and rivers do not suddenly freeze in the winters.
(d) The solute cannot be separated from a Solution by filtration.
(e) Fused CaCl2 or conc. H2SO4 is used in a desiccator.
(f) Effervescence is seen on opening a bottle of soda water.
(g) Table salts become sticky on exposure to humid air during the rainy season.
Answer:
(a) Water is an excellent liquid to use in cooling systems because of its high specific heat.
(b) A water-soluble solid disappears in a Solution where the solvent is water, and water has the property of being clear and transparent. So, the Solution is also clear and transparent.
(c) Lakes and rivers do not freeze suddenly in winters
because of the high specific latent heat of solidification, i.e. the amount of heat released when 1 g of water solidifies to form 1 g of ice at 0°C. It is about 336 J/g or 80 cal/g.
(d) The component which dissolves in a solvent is known as a solute. So, it cannot be separated from a Solution by filtration. However, filtration is used when the solute is insoluble in the Solution.
(e) Fused CaCl2 or concentrated H2SO4 is deliquescent in nature, i.e. it absorbs moisture, and hence, these are used in desiccators as drying agents.
(f) Carbon dioxide is dissolved in soda water under pressure. On opening the bottle, the pressure on the surface of water suddenly decreases; therefore, the solubility of CO2 in water decreases and the gas rapidly bubbles out.
(g) Table salt becomes sticky on exposure during the rainy season, because it generally contains a small percentage of magnesium chloride and calcium chloride as impurities. These impurities absorb moisture from the monsoon air due to their deliquescent nature, and thus, table salt become sticky.
(Water Exe-3B Chemistry Class-9 ICSE)
Question 14.
Normally, solubility of crystalline solid increases with temperature. Does it increase uniformly in all cases? Name a substance whose solubility:
(a) Increases rapidly with temperature.
(b) solubility Increases gradually with temperature.
(c) Increases slightly with temperature.
(d) Initially increases then decreases with rise in temperature.
Answer:
(a) Potassium nitrate
(b) Potassium chloride
(c) Sodium chloride
(d) Calcium sulphate
Question 15.
What are drying or desiccating agents? Give examples.
Answer:
These are substances which can readily absorb moisture from other substances without chemically reacting with them.
Examples:
Phosphorous pentoxide (P2O5), quick lime (CaO)
Question 16.
Complete the following table:
| Common Name | Chemical Name | Formula | Acid, base or salt | Efflorescent,
hygroscopic or deliquescent substance |
| Solid caustic potash | ||||
| Quick lime | ||||
| Oil of vitriol | ||||
| Washing soda | ||||
| Solid caustic soda | ||||
| Blue vitriol |
Answer:
| Common Name | Chemical Name | Formula | Acid, base or salt | Efflorescent,
hygroscopic or deliquescent substance |
| Solid caustic potash | Potassium hydroxide | KOH | Base | Deliquescent substance |
| Quick lime | Calcium oxide | CaO | Base | Hygroscopic substance |
| Oil of vitriol | Sulphuric acid | H2SO4 | Acid | Hygroscopic substance |
| Washing soda | Hydrated sodium carbonate | Na2CO3.10H2O | Salt | Efflorescent substance |
| Solid caustic soda | Sodium hydroxide | NaOH | Base | Deliquescent substance |
| Blue vitriol | Copper sulphate | CuSO4 | Salt | Efflorescent substance |
Question 17.
In which of the following substances will there be
(a) Increase in mass
(b) Decrease in mass
(c) No change in mass when they are exposed to air?
- Sodium chloride
- Iron
- Conc. sulphuric acid
- Table salt
- Sodium carbonate crystals
Answer:
(a) Increase in mass: Iron and conc. sulphuric acid
(b) Decrease in mass: Sodium carbonate crystals
(c) No change in mass: Sodium chloride
Question 18.
State the methods by which hydrated salts can be made anhydrous.
Answer:
Hydrated salts can be converted to anhydrous substances by heating and also when exposed to dry air.
Example:
Gaber’s salt becomes powdery anhydrous sodium sulphate when exposed to dry air.

— : End of Water Exe-3B Answer Class-9 ICSE Chemistry Solutions :–
Return to Return to Concise Selina ICSE Chemistry Class-9
Thanks
Please share with your friends