Water New Simplified Class-9 Dalal ICSE Chemistry Solutions Chapter-3. We Provide Step by Step Solutions of Exercise/Lesson -3 Water with Additional Questions , Previous Year Questions and Unit Test-3 of Dr Viraf J Dalal Middle School Chemistry Allied Publishers. Visit official Website CISCE for detail information about ICSE Board Class-9.
Water New Simplified Class-9 J Dalal ICSE Dr Viraf Chemistry Solutions Chapter-3
–: Select topics :–
Previous Year Questions of Chapter-Water for ICSE Class-9 Chemistry
Question 1.(1984)
Name one substance which is ‘deliquescent’.
Answer:
Deliquescent substance is NaOH (sodium hydroxide), KOH, CaCl2
Question 2.(1984)
How does an increase in temperature affect :
- the solubility of NaCl
- the solubility of CaSO4 in water ?
Answer:
Increase in temperature :
- Increases the solubility of NaCl only a little.
- Solubility of CaSO4 first increases and then decreases.
Question 1.(1985)
Give reasons for the following :
- table salt becomes moist and sticky during the rainy season
- a white power forms on the surface of washing soda crystals which are left exposed to the air.
Answer:
- Table salt contains impurities of calcium and magnesium chloride which are Deliquescent and absorb moisture from air and common salt becomes Sticky and Wet During humid conditions [rainy season].
- Washing Soda is Efflorescent substance when exposed to air loses its moisture [water of crystallisation partly and becomes powder which forms white powder on the crystals.
Question 1.(1986)
Name a salt
(a) which contain of crystallization
(b) which does not contain.
Answer:
(a) Blue vitriol [copper sulphate] CuSO4. 5H2O
(b) Potassium chloride KCl or sodium chloride NaCl
Question 1.(1987)
Name a deliquescent substance’.
Answer:
Iron [III] chloride [FeCl3]
Question 1.(1988)
Explain the following observations :
- Washing-soda become coated with a white powder when left exposed to the atmosphere.
- In the expression anhydrous copper sulphate, what is meant by “anhydrous”.
- Why is fused calcium chloride or cone, sulphuric acid used in a desiccator.
Answer:
- Washing Soda : is efflorescent substance and lose its water of crystallisation partly when exposed to atmosphere and change into white powder (amorphous state).
- Copper sulphate loses water of crystallization when heated and becomes white powder of CuSO4. This white powder (CuSO4) without water is anhydrous.
- Fused CaCl2 or cone. H2SO4 is used in desiccator as drying agent to remove moisture from the substance (to dry it) but does not change its composition.
Question 2.(1988)
Complete the following : The solubility of a gas at constant pressure may be increased by decreasing the
Answer:
The solubility of a gas at constant pressure may be increased by decrease in temperature.
Question 1.(1991)
What is ‘water of crystallization’ ? Name a crystalline salt which does not contain water of crystallization.
Answer:
Water Of Crystallization : “The fixed number of water molecules which enter into a loose Chemical Combination with the substance when the substance is crystallised from its hot saturated solution is called water of crystallization.”
The substance is crystalline but does not contain water of crystallization is KCl
Question 2.(1991)
What would you observe, when the water of crystallization of a salt Is removed by heating it.
Answer:
It will turn Amorphous in nature lose its Geometric shape, become powder.
Question 3.(1991)
Define :
- Hygroscopy
- Efflorescence.
Answer:
- Hygroscopy : “is the phenomenon of a subtance to absorb water (moisture) from the atmosphere but does not change its state.”
- Efflorescence : “Is the phenomenon in which a substance loses it moisutre (water of crystallisation partly or completely to atomsphere and change to amorphous state, when exposed to atmosphere.”
Question 4.(1991)
What is the effect of temperature on the solubility of KNO, and calcium sulphate in water.
Answer:
Solubility of KNO3 increases with temperature and solubility of calcium sulphate first increases upto 50°C and then decreases upto 100°C.
Question 1.(1992)
What test would you do to find out whether a given solution is saturated or unsaturated.
Answer:
If on adding more of solute in the given solution and stirring, the solute dissolves, it is unsaturated solution. If the solute settles down and does not dissolve, it is saturated solution.
Question 2.(1992)
How can you increase the solubility of a given volume of gas in water.
Answer:
Solubility of a given volume of gas can be increased by increasing the pressure on the surface of water.
Question 1.(2007)
Define ‘eutrophication’.
Answer:
Eutrophication : “Organic matter in sewage poured into water bodies generally results in excessive growth of algae – which deoxygenates water and produces deadening atmosphere there.”
Question 2.(2007)
What is meant by the term ‘oil spill’.
Answer:
Oil spill or Leaks : Oil is lighter and insoluble in water and oil layer floats on the surface of water and prevents oxygen transfer from atmosphere.
Oil leakage and petroleum products into sea water due to accidents of ships and oil tankers or leakage of pipe lines and storage tanks. They occur when oil is being produced from offshore well and may happen due to transportation by pipes and tanks, oil refineries, petrochemical plants etc.
Damage caused by oil spills :
- Fish, birds, reptiles and amphibians living in such water cannot breathe and die. The oil penetrates the birds feathers and they cannot fly, and affect their insulation and damages reproductive system.
- It interrupts the food chain which may cause extinction of species.
Question 1.(2008)
State any two sources of water pollution.
Answer:
Two sources of water pollution are :
(a) Industrial waste
(b) Sewage.
Question 2.(2008)
State the causes and consequences of ‘eutrophication’.
Answer:
Causes of eutrophication are :
(a) Increase in chemical nutrients in an ecosystem.
(b) Organic matter in sewage poured into water bodies.
Question 3.(2008)
What is meant by the term ‘offshore drilling’. State the main environmental effects of offshore drilling.
Answer:
Offshore drilling : Involves exploring for oil and gas beneath the ocean floor. Steps involved are location of wells, exploring wells to find out if there is oil below and if oil and gas ‘production well’ is drilled and an ‘oil rig’ is built to replace the exploratory drilling rig.
Environmental effects : It affects the health and reproduction of marine animals. It interrupts the food chain, which may cause extinction of species.
Question 4.(2008)
Explain why oil spills have an adverse effect on marine life.
Answer:
Oil is lighter and insoluble in water and oil layer floats on the surface of water and prevents oxygen transfer from atmosphere.
Oil leakage and petroleum products into sea water due to accidents of ships and oil tankers or leakage of pipe lines and storage tanks. They occur when oil is being produced from offshore well and may happen due to transportation by pipes and tanks, oil refineries, petrochemical plants etc.
Damage caused by oil spills :
- Fish, birds, reptiles and amphibians living in cannot breathe and die. The oil penetrates the birds feathers and they cannot Tly, and affect their insulation and damages reproductive system.
- It interrupts the food chain which may cause extinction of species.
Question 1.(2009)
Explain any two environmental impacts of an ‘oil spill’.
Answer:
See Question 2, 2007.
Question 1.(2010)
Explain the methods of controlling water pollution.
Answer:
Methods of controlling water pollution.
- Control of water pollution can be done by properly handling of sewage waste.
- Use drilling fluids which are biodegradable and have low aquatic toxicty.
- Develop better pollution control measures which include removal of oil, dispersing oil, removing of oil clumps.
- Oil and grease is removed from industrial waste by oil-water separator.
- Acid and alkalies are neutralized.
- Organic materials in waste water are removed by distillation adsorption.
- Biodegradable organics are removed by (a) Activated sludge process (an aerobic biochemical process) (b) Biological trickling filter process.
Additional Questions of Exercise-Water Dalal New Simplified Chemistry
Question 1.
State the importance of water for all general uses.
Answer:
Importance of water : Water is vital for the growth of plants and animal life. Water is also essential for industrial processes, agricultural processes transportation and for power generation and cleanliness.
Question 2.
How does it occur in the free state and in the combined state.
Answer:
Water occurs in free state : In the form of ice, snow, frost
As – river water, lake water, sea water, spring water
As – water vapour, clouds, mist, fog
In combined state : In plants and animals, in hydrated salts i.e., MgCl2.6H2O and in certain minerals, milk, dry cereals and in green vegetables.
Question 3.
State a reason to prove that water is a compound and not a element.
Answer:
Three reasons are :
- Water has new properties than its constituents [i.e. H2 and O2]. H2 is combustible gas, O2 gas is supporter of combustion but water is liquid.
- In water
- hydrogen and oxygen elements combine in fixed ratio by
- Components of water i.e. H2 and oxygen can be separated by chemical means e.g. by electrolysis of water.
Question 4.
State why ‘Water is considered a universal solvent’. Give the reason for the same.
Answer:
Water is universal solvent : Water dissolves many substances forming aqueous solutions. Not only solids, but gases and other liquids also dissolve in water. When water is put in the glass vessel an extremely small amount of glass dissolves in it. Organic compounds [carbohydrates, proteins] also dissolve in water. Hence water is called universal solvent.
Question 5.
Define the terms :
- solute
- solvent
- solution
Answer:
- Solute : In a salt solution, salt is added to water then salt [NaCl] is solute i.e. substance dissolves.
- Solvent : Medium (liquid) in which solute is dissolved i.e. water is solvent to which salt dissolves to form salt solution.
- Solution : “A homogenous mixture of a solute in a solvent” is called solution.
Question 6.
State the characteristics of a true solution.
Answer:
Characteristics of a true solutions are :
- Is homogenous in nature.
- (a) Particles can pass through the filter paper, (b) Cannot be seen under microscope, (c) Do not settle down.
- A true solution is a mixture and not a compound.
Question 7.
Differentiate between unsaturated, saturated & supersaturated solutions.
Answer:
- Saturated : Solution cannot dissolve any more of solute at a given temperature.
- Unsaturated : solution can dissolve more solute at a given temperature.
- Supersaturated : Solution which has more solute than its saturated sol. at that temperature.
Question 8.
How would you convert a saturated solution to an unsaturated solution and vice versa.
Answer:
- A saturated solution can be made unsaturated by adding more of solvent.
- An unsaturated solution can be made saturated by adding more of solute.
Question 9.
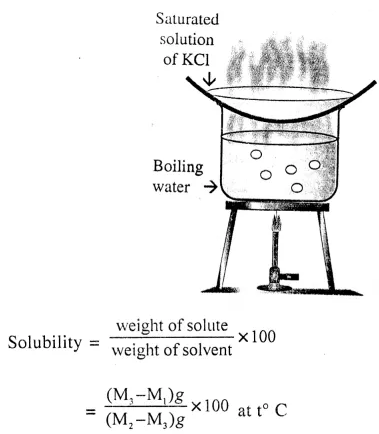
Define solubility. Give the main steps with the calculations involved of the method to determine the solubility of a given salt ‘X’ in water.
Answer:
Solubility : “The maximum amount of a SOLUTE which can be dissolved in 100 grams of a solvent at a specified temperature is called Solubility.
To Determine The Solubility Of ‘X’ In Water :
Steps :
(i) Preparation of saturated solution of ‘X’
- Take 100 ml of distilled water in a boiling test tube.
- Add crystals of ‘X’ to distilled water and stir slowly.
- Continue adding and ktirring till the crystals dissolve. Repeat the process till no more salt can dissolve.
- Pour the saturated solution — in a clean dry test-tube.
Steps :
(ii) Calculations To Determine Solubility Of Solute :
- Weigh a clean and dry vaporating dish = M1g
- Add above saturated solution to it and weigh = M2g
∴ Weight of solution (solute + solvent water) = (M2 – M1) g - Heat the solution to dryness and weigh the dish with residue = M3g
Weight of solute = (M3 – M1) g
weight of solvent (water) = M2 – M3 = solution – solute (M2 – M1) = (M3 – M1) = M2 – M1 – M3 + M1 - Note the temperature of saturated solution = t° C
Question 10.
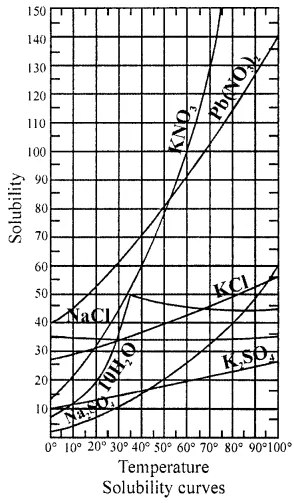
From the following list of salts : Na2SO4, 10H2O, NaCl, KClO3, NaNO3, Ca(OH)2, NH4Cl, KCI, CaSO4.
State the salts whose solubility (a) increases, (b) decreases, (c) is fairly independent or slightly increases – with rise in temperature of water.
Answer:
(a) Solubility Increase with rise of temperature KClO3, NH4Cl
(b) Decreases with rise in temperature CaSO4, Ca(OH)2 above 70° C
(c) Slightly increases with rise in temperature
NaCl, KCl, Ca(OH)2 below 70°C
Question 11.
What is a solubility curve. State two applications and two benefits of the solubility curve.
Answer:
Solubility curve : “The effect of temperature on solubility of solute in a solvent shown by a curve in the graph (temperature-solubility) is called solubility curve.”
Two applications of the solubility curve :
- MEDICAL : Enables a pharmacist to determine the amount of drugs that must be dissolved together in a given quantity of solvent at different temperatures to give a prescribed drug preparation.
- Chemists And Research Workers : Helps them to determine the most suitable solvent to be used at various temperatures for Extraction Of Essential Chemicals from their natural sources.
Two benefits of the solubility curve :
- To compare solubilities of different solutes in a solvent at a given temperature.
- To determine solubility of a given solute at a specific temperature.
Question 12.
Give the influence of
- pressure
- temperature on the solubility of gases in water.
Answer:
Influence on solubility of gases :
- Pressure : “is directly proportional to pressure” at a given temperature. Solubility of gases increases with increase in pressure and decreases with decrease in pressure.
- Temperature : “is inversely proportional to temperature”, i.e. increase in temperature of water causes decrease in solubility of gases. At low, temperature the solubility of the gas is more compared to higher or ordinary temperature.
Question 13.
State the reasons why
- boiled water tastes flat
- a soda water bottle opens with a ‘fizz’.
Answer:
- Boiled Water Tastes Flat : Soluble gases in water contribute to the taste of water.
When water is boiled Solubility Of Gases decrease and gases conic out of water and water tastes flat. - Soda Water Bottle Opens With A ‘Fizze’.
An unopened soda bottle is virtually bubble-free because the pressure inside the bottle keeps the carbon dioxide dissolved in the liquid. When you crack open the bottle, and allow the gas bubbles to wiggle the pressure is released and allow the gas bubbles to wiggle free from the liquid and rise to the surface.
Question 14.
What is meant by the terms :
(a) crystal,
(b) crystallization,
(c) seed crystal.
Explain with examples
Answer:
(a) Crystal : “On cooling a Hot Saturated Solution, homogenous solids arranged symmetrically are obtained, called Crystals.”
(b) Crystallization : “The process by which crystals are separated or deposited from a hot saturated solution of a substance on cooling gently is called crystallization.”
(c) Seed crystal : “A well-formed crystal from the cooled filtrate of saturated solution used as seed for the formation (growing) of large sized crystal is called SEED CRYSTAL.”
e.g. a large size crystal of potassium nitrate is prepared from saturated solution of KNO3 saturated solution.
Question 15.
Define the term ‘water of crystallization’.
Answer:
Water Of Crystallization : “Is The Fixed Number Of Water Molecules Which Enter Into A Loose Chemical Combination With The Substance When The Substance Is Crystallized From Its Hot Saturated Solution, Is Called Water Of Crystallization.”
Question 16.
Differentiate between hydrated and anhydrous crystals with examples. State three defined changes which occur when hydrated copper sulphate is heated.
Answer:
Difference between hydrated and anhydrous crystals :

- Colour changes from blue to colourless.
- Changed to AMORPHOUS white powder
- Geometric shape vanishes
- On cooling and adding water colour restores but crystalline does not shape.
Question 17.
Washing soda and iron [III] chloride are separately exposed to the atmosphere. State
- the observations seen
- the phenomenon which occurs
- the reason for the phenomenon occurring in each case.
Would a similar phenomenon occur in case of exposure of common salt. Explain giving reasons.
Answer:
Observations When Exposed To Atmosphere :
(i) Washing soda :
(a) Loses its moisture (water of crystallization)
(b) Becomes amorphous
(c) Coated with white powder
(ii) Iron (III) Chloride (FeCl2)
Absorbs moisture from atmsophere dissolves in moisture changes to Liquid state
(iii) In case of washing soda — the phenomenon is Efflorescence.
In case of FeCl3 it is Deliquescence
No, in case of common salt (NaCl) no such phenomenon can occur as, it is Anhydrous salt and does not contain water of crystallization.
When common salt contains impurities of calcium or magnesium chloride dilquescent it absorbs moisture from atmosphere and become sticky and wet.
Question 18.
Why is fused calcium chloride and not potassium chloride kept in a desiccator?
Answer:
Fused Calcium Chloride (CaCl2) Is Deliquescent in nature, absorbs moisture and hence is used as Drying Agent in desiccator for drying other substances in the laboratory but KCl (potassium chloride) has no such property and cannot be used.
Question 19.
How does fused calcium chloride differ from iron [III] chloride when exposed to the atmosphere?
Answer:
Fused CaCl2 is Hygroscopic substance, absorbs moisture from atmosphere like FeCl3 but does not change its state. FeCl3 on absorbing moisture changes to Liquid State.
Question 20.
Cone. H2SO4 acts as a ‘drying agent’ & a ‘dehydrating agent’. Explain and differentiate the words in italics.
Answer:
Cone. H2SO4 when acts as drying agent, it absorbs only moisture from the substance and makes it dry without changing its composition.
When cone. H2SO4 acts as dehydrating agent, it removes water molecule from the composition of substance and reacts chemically, produces a new substance with new properties.
Question 21.
Explain the meaning of the terms – hard water & soft water.
Answer:
- Hard Water : “Water that does not lather readily-with ordinary soap and hence wastes soap” is called hard water.
- Soft Water : “Water that latters readily with ordinary soap and — hence soap is not wasted” is called soft water.
Question 22.
State the causes of hardness in water.
Answer:
Presence of salts of calcium and magnesium i.e. Ca(HCO3) or Mg(HCO3) or calcium or magnesium sulphate or chloride in water are the Causes Of Hardness Of Water.
Question 23.
Give two natural sources of hard water.
Answer:
Two natural sources of hard water :
- Water from springs
- River water.
Question 24.
Differentiate between temporary hard water & permanent hard water.
Answer:
Differences between temporary hard water and permanent hard water :
Temporary hard water :
- is hard due to the presence of Bicarbonates of Calcium or magnesium.
- Hardness can be removed by boiling.
Permanent hard water :
- is hard due to the presence of salts like chlorine or sulphate of calcium or magnesium.
- Hardness cannot be removed by boiling.
Question 25.
State the cause of hardness in temporary & permanent hard water.
Answer:
Cause Of Hardness in Water :
- Temporary hard water — bicarbonate of calcium or bicarbonate of magnesium.
- Permanent hard water — CaCl2 or CaSO4 or MgCl2 or MgSO4
Question 26.
State the disadvantages of hardness in water.
Answer:
Disadvantages of hardness in water :
- Soap is wasted and clothes do not get clean.
- Unsafe for drinking.
- Unfit for laundries.
- Forms boiler scale or fur in boilers.
- Not suitable for preparing solutions.
Question 27.
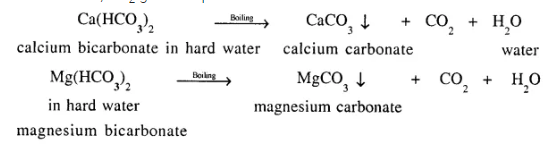
Temporary hardness in water can be removed by boiling. Give balanced equations to explain how, hardness in temporary hard water is removed by boiling.
Answer:
Temporary hardness in water which is due to the presence of HCO3 of calcium or magnesium.
On boiling bicarbonate changes to carbonate which is insoluble in water and is filtered out, CO2 gas escapes and water becomes soft.
Question 28.
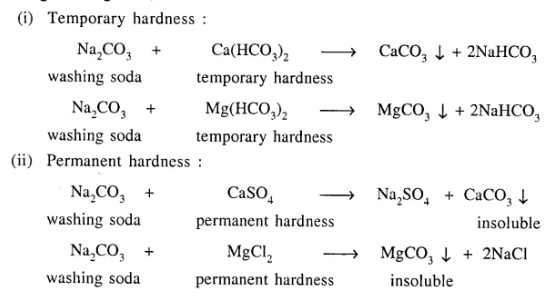
Both temporary & permanent hardness in water can be removed by addition of washing soda. Give balanced equations for the same.
Answer:
Using washing soda, removal of hardness of water :
Question 29.
A sample of water is given in a trough. State how would you prove experimentally whether the given sample is hard water or soft water.
Answer:
On adding ordinary soap in the sample and shaking if this water gives lather easily it is soft water otherwise it is hard water.
Question 30.
Two samples of water are placed in a beaker individually. State how you will determine experimentally which of the two samples contains permanent hard water.
Answer:
Boil each sample of water separately and then two each sample add ordinary soap, the sample that lathers is temporary hard water and the sample which does not lather is permanent hard water.
Question 31.
State what are synthetic detergents. Explain experimentally how you will determine the advantage of synthetic detergents over soap using a sample of hard water.
Answer:
Divide the sample of hard water into two parts.
To the first part of hard water add synthetic detergent and rub slowly with hand, it will lather easily.
Now add to the second part of hard water an ordinary piece of soap and rub with hand, it will not lather but scum is formed and soap is wasted.
Hence in case of hard water, it is advantageous to use synthetic detergent.
Water – Unit Test Paper 3 ( New Simplified Dalal ICSE Class-9)
Q.1. Select the correct word from the words in brackets to complete each sentence :
Question 1.
If pressure on the surface of water increases its boiling point ____ and freezing point ____ [increases / decreases].
Answer:
If pressure on the surface of water increases its boiling point increases and freezing point decreases.
Question 2.
A saturated solution can be converted to an unsaturated solution by ____ [increasing / decreasing] the amount of the solvent.
Answer:
A saturated solution can be converted to an unsaturated solution by increasing the amount of the solvent.
Question 3.
Dissolved air in water contains a ____ [higher / lower] percentage of oxygen than ordinary air.
Answer:
Dissolved air in water contains a higher percentage of oxygen than ordinary air.
Question 4.
At low temperatures the solubility of a gas in water is ____ compared to that at ordinary temperatures.
Answer:
At low temperatures the solubility of a gas in water is more compared to that at ordinary temperatures.
Question 5.
Efflorescence occurs when the vapour pressure of the hydrated crystals is ____ (more/less) than the vapour pressure of the atmospheric humidity.
Answer:
Efflorescence occurs when the vapour pressure of the hydrated crystals is more than the vapour pressure of the atmospheric humidity.
Q.2. Select the correct answer from the choice given in the brackets.
Question 1.
An anhydrous crystal, (blue vitriol/epsom salt/lead chloride).
Answer:
An anhydrous crystal lead chloride.
Question 2.
A substance which causes hardness in water. (NH4Cl/CaCl2/NaCl)
Answer:
A substance which causes hardness in water CaCl2.
Question 3.
A deliquescent salt of a divalent metal. (CuCl/CaCl2/FeCl2/PbCl2)
Answer:
A deliquescent salt of a divalent metal CaCl2.
Question 4.
An anhydrate of a heptahydrate salt. (Cu(N03)2/Ca(NO3)2/FeSO4/CaSO4)
Answer:
An anhydrate of a heptahydrate salt FeSO4
Question 5.
A drying agent, deliquescent in nature used in a dessicator. (cone. H2SO4/ fused CaCl2FeCl3)
Answer:
A drying agent, deliquescent in nature used in a dessicator fused CaCl2.
Q.3. Give reasons for the following (Water – Unit Test Paper 3)
Question 1.
Solubility curves find utility in separation and purification of solutes.
Answer:
Those fractions with very low solubilities will be the first to crystallise out from the solution and will be separated out.
Question 2.
Pressure and temperature influence the solubility of gases in water.
Answer:
- An Increase In Pressure On The Surface Of Water Causes Increase In Solubility Of Gas In Water.
- An Increase In Temperature Of Water Causes Decreas In Solubility Of Gas In Water.
Question 3.
Heating a hydrated copper sulphate cryttal is deemed a chemical change.
Answer:
Heating hydrated copper sulphate crystal is a chemical change as on adding water drops on white powder of CuSO4 (amorphous) colour restores but geometric shape is not restored i.e. after heating new composition is found to give crystalline shape.
Question 4.
Efflorescence is minimum during humid conditions.
Answer:
Efflorescence Occurs : When The Vapour Pressure Of Hydrated Crystals Exceeds The Vapour Pressure Of The Atmospheric Humidity.
Hence efflorescence is minimum during humid conditions.
Question 5.
A crusty ‘boiler scale’ is formed in boilers, when hard water is used.
Answer:
A crusty ‘boiler scale’ is formed in boilers, when hard water is used because insoluble CaCO3 or MgCO3 is formed in large scale which gets deposited as crusty ‘boiler scale’. Its formed when bicarbonates of calcium or magnesium are converted into carbonates.
Q.4. Name or state the following.
- An efflorescent decahydrate salt.
- A deliquescent salt of a trivalent metal.
- A liquid hydroscopic substance.
- A salt whose solubility decreases with rise in temperature of the solvent water.
- A substance added to remove both temporary and permanent hardness in water.
Answer:
- An efflorescent decahydrate salt is washing soda (Na2CO3.10H2O)
- A deliquescent salt of a trivalent metal is iron (III) chloride (FeCl3)
- A liquid hygroscopic substance — concentrated sulphuric acid (cone. H2SO4)
- CaSO4
- Washing soda (Na,CO3)
Q.5. Differentiate between the following :
- Natural water and treated water
- Saturated solution and a supersaturated solution
- Solubility and solubility curve
- Deliquescent salt and hygroscopic salt
- Solute and solvent – forming a solution.
Answer:
Difference between :
1. Natural water and treated water :
-
- Natural water : Water obtained in nature, river water, lake water, sea water, snow, rain water.
- Treated water : Water which has received some form of treatment – ice, mineral water, distilled water, water in swimming pool.
2. Saturated solution and a supersaturated solution :
- Saturated : Solution cannot dissolve any more of solute at a given temperature.
- Supersaturated : Solution which has more solute than its saturated sol. at that temperature.
A saturated solution can be made unsaturated by adding more of solvent.
3. Solubility :
(a) Is the ability of solute to dissolve in a particular solvent
(b) It is used to find the amount of solute required.
Solubility curve :
(a) Is a line graph that plots the changes in the solubility of a solute in a solvent against changes in temperature
(b) It is used to compare the solubility of different solutes.
4. Deliquescent salt :
“When exposed to atmosphere lose (moisture) water of crystallization partly or wholly and become amoi’phous.” e.g. washing soda (Na2CO3.10H2O).
Hygroscopic salt :
“absorb water or moisture from the atmosphere when exposed but donot change their state like deliquent salts.” e.g. Cone. H2SO4, quick lime CaO.
5. Solute :
(a) It is the substance which is dissolved to form solution
(b) It is small in quantity.
Solvent :
(a) It is medium in which solute is dissolved
(b) It is large in quantity.
Q.6. Match the terms in List I with the correct answers in List II. (Unit Test-3 Water Dalal)

.– : End of Water Solutions :–
Return to New Simplified Dalal ICSE Chemistry Class-9 Solutions
Thanks
Share with your friends

Thank you for all the answers..i really appreciate it
thanks for positive response
There is something wrong some questions are not matching to book…its may be wrong aur you have written right answer but
we will update soon
Extremely helpful! Thanks a lot!
keep in touch