Chemical Changes and Reactions Exe-2B Chemistry Class-9 ICSE Selina Publishers Solutions Chapter-2. Step By Step ICSE Selina Concise Solutions of Chapter-2 Chemical Changes and Reactions with All Exercise including MCQs, Very Short Answer Type, Short Answer Type, Long Answer Type, Numerical and Structured/Application Questions Solved . Visit official Website CISCE for detail information about ICSE Board Class-9.
Chemical Changes and Reactions Exe-2B Chemistry Class-9 ICSE Concise Selina Publishers
| Board | ICSE |
| Publications | Selina Publication |
| Subject | Chemistry |
| Class | 9th |
| Chapter-2 | Chemical Changes and Reactions |
| Book Name | Concise |
| Topics | Solution of Exercise – 2B |
| Academic Session | 2023-2024 |
B. Exercise – 2B
Chemical Changes and Reactions Class-9 Chemistry Concise Solutions
Page 30
Question 1.
Choose the correct answer from the options give below.
(a) Which of the following is not a characteristic of a chemical change?
(i) It is irreversible.
(ii) No net energy change is involved.
(iii). New substance is formed.
(iv)Involves absorption or liberation of energy.
(b) A reaction of a type: AB + CD → AD + CD, involves
(i) No chemical change
(ii) Decomposition of AB and CD
(iii) . Exchange of ions of AB and CD
(iv) Combination of AB and CD
(c) The reaction BaCl2(aq)+ H2SO4(aq) → BaSO4(s) + 2HCl(aq) is
(i) Displacement reaction
(ii) Neutralisation reaction
(iii) . Decomposition reaction
(iv) Double displacement reaction
(d) Thermal decomposition of sodium carbonate will produce
(i) Carbon dioxide
(ii) Oxygen
(iii) Sodium hydroxide
(iv) No other product
Answer:
(a) (ii) No net energy change is involved.
(b) (iii) Exchange of ions of AB and CD
(c) (iv)double displacement reaction
(d) (i) Carbon dioxide
Question 2.
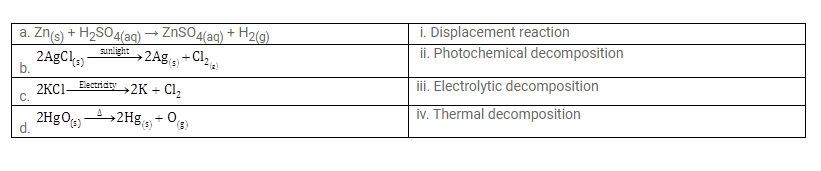
Match the following: (See text book for Question)
| a…….. | i. Photochemical decomposition |
| b. ………. | ii. Thermal decomposition |
| c. ………… | iii. Displacement reaction |
| d. ……….. | iv. Electrolytic decomposition |
Answer:
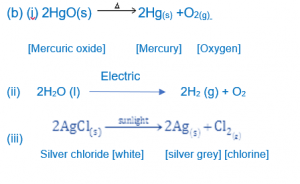
Question 3.
Complete the following statements:
(a) The chemical change involving iron and hydrochloric acid illustrates a _________________ reaction.
(b) In the type of reaction called_______________, two compounds exchange their positive and negative radicals.
(c) A catalyst either ______ or _____________ the rate of a chemical change but itself remains ______________ at the end
Of the reaction.
(d) On heating, hydrated copper sulphate changes its colour from ________ to __________.
Answer:
(a)Displacement
(b)Double decomposition
(c)Accelerates, decelerates, unaffected
(d)Blue, white
Question 4.
When hydrogen burns in oxygen, water is formed; when electricity is passed through water, hydrogen and oxygen are given out. Name the type of chemical change involved in the two cases.
Answer:
When hydrogen burns in oxygen, water is formed – Combination Reaction.
When electricity is passed through water, hydrogen and oxygen are given out – Decomposition Reaction.
Question 5.
Explain, giving one example for each of the following chemical changes:
(a) Double decomposition
(b) Thermal decomposition
(c) Reversible reaction
(d) Displacement
Answer:
(a) Double decomposition reaction
This is a type of chemical change in which two compounds in a Answer react to form two new compounds by mutual exchange of radicals
(b) Thermal decomposition
A decomposition reaction brought about by heat is known as thermal decomposition.
2HgO(s) 2Hg(s) +O2(g)
(c) Reversible reaction
A chemical reaction in which the direction of a chemical change can be reversed by changing the conditions under which the reaction is taking place is called a reversible reaction.
CuSO4.5H2O(s) ⇋ CuSO4(s) + 5H2O (g)
(d) Displacement Reaction
It is a chemical change in which a more active element displaces a less active element from its salt solution.
CuSO4 + Zn → ZnSO4 + Cu
Question 6.
(a) What is synthesis?
(b) What kind of chemical reaction is synthesis? Support your answer by an example.
Answer:
A reaction in which two or more substances combine together to form a single substance is called a synthesis or combination reaction.
A + B → AB
In the above reaction, substances A and B combine to give a molecule of a new substance, AB.
Carbon burns in oxygen to form a gaseous compound, carbon dioxide.
C + O2 == CO2
B. Exercise – 2B
Chemical Changes and Reactions Class-9 Chemistry Concise Solutions
Page 31
Question 7.
Decomposition brought about by heat is known as thermal decomposition. What is the difference between thermal dissociation and thermal decomposition?
Answer:
A decomposition reaction brought about by heat is known as thermal decomposition.
2HgO(s) —- 2Hg(s) + O2 (g)
A simultaneous reversible decomposition reaction brought about only by heat is thermal dissociation.
NH4Cl ⇋ NH3 +HCl
Question 8.
(a) Define neutralization reaction with an example.
(b) Give balanced equation for this reaction.
(c) Give three applications of neutralization reactions.
Answer:
(a)The reaction between an acid and a base to form salt and water only is referred to as a neutralisation reaction.
(b)NaOH + HCl → NaCl + H2O
(c) Applications of neutralisation reactions:
(i) When someone is stung by a bee, formic acid enters the skin and causes pain, which can be relieved by rubbing the spot with slaked lime or baking soda, both of which are bases.
(ii) Acid which is accidentally spilled on to our clothes can be neutralised with ammonia solution.
(iii) If soil is somewhat acidic and thus unfavourable for growing of certain crops, slaked lime is added to neutralise the excess acid.
Question 9.
What do you understand by precipitation reaction? Explain with an example.
Answer:
A chemical reaction in which two compound in their aqueous state react to form an insoluble salt (a precipitate) as one of the product is known as precipitation reaction.
For example: BaCl2 (aq) +NaSO4 (aq) →BaSO4(s) white ppt + 2NaCl (aq).
Question 10.
(a) What are double displacement reactions?
(b) Give an example of double displacement reaction, where a gas is evolved.
Answer:
(a)This is a type of chemical change in which two compound in a solution react to form two compound by mutual exchange of radicals Double decomposition reaction is also called double displacement reaction.
AB +CD→ AD +CB
(b) Double displacement reaction, gas evolved are
FeS(s) + H2SO4 (aq) → FeSO4 (aq) + H2S
Question 11.
(a) What is a decomposition reaction?
(b) Decomposition reaction can occur by (i) heat
(ii) Electricity and (iii) sunlight
Give two balanced reaction for reaction
Answer:
(a)The chemical reaction in which a compound splits into two or more simpler substance (elements or compound) is called decomposition reaction.
Question 12.
State the type of reactions each of the following represent and balance the ones that are not balanced.
(a) Cl2 + 2KBr → 2KCl + Br2
(b) NaOH + HCl → NaCl + H2O
(c) 2HgO → 2Hg + O2
(d) Fe + CuSO4→ FeSO4 + Cu
(e) PbO2 + SO2→ PbSO4
(f) 2KClO3→ 2KCl + 3O2
(g) 2H2O2→ 2H2O + O2
(h) KNO3 + H2SO4 → HNO3 + KHSO4
(i) CuO+H2→ Cu+ H2O
(j) CaCO3→ CaO+ CO2
(k) NH4Cl → NH3 + HCl
(l) PbO + 2HNO3→ Pb(NO3) + 2H2O
(m) AgNO3 + NaCl → AgCl + NaNO3
Answer:
(a) Cl2 + 2KBr → 2KCl + Br2
Displacement reaction
(b) NaOH + HCl → NaCl + H2O
Neutralisation reaction
(c) 2HgO → 2Hg + O2
Decomposition reaction
(d) Fe + CuSO4 → FeSO4 + Cu
Displacement reaction
(e) PbO2 + SO2 → PbSO4
Combination reaction
(f) 2KClO3 → 2KCl + 3O2
Decomposition reaction
(g) 2H2O2 → 2H2O + O2
Decomposition reaction
(h) KNO3 + H2SO4→ HNO3 + KHSO4
Double decomposition reaction
(i) CuO+H2 → Cu+ H2O
Displacement reaction
(j) CaCO3 → CaO+ CO2
Decomposition reaction
(k) NH4Cl → NH3 + HCl
Decomposition reaction
(l) PbO + 2HNO3 → Pb(NO3) + 2H2O
Neutralisation reaction
(m) Double decomposition reaction
AgNO3 + NaCl → AgCl + NaNO3
— : End of Chemical Changes and Reactions Exe-2B Answer Class-9 ICSE Chemistry Solutions :–
Return to Return to Concise Selina ICSE Chemistry Class-9
Thanks
Please share with your friends


