Exe-12B Radioactivity ICSE Class-10 Concise Physics Selina Solutions Chapter-12. We Provide Step by Step Answer of Exercise-12(A), MCQs-12(A),, Exercise-12(B), MCQ-12(B) and Numericals -12(B) Questions of Exercise-12 Radioactivity ICSE Class-10 Concise . Visit official Website CISCE for detail information about ICSE Board Class-10.
| Board | ICSE |
| Publications | Selina Publication |
| Subject | Physics |
| Class | 10th |
| Chapter-12 | Radioactivity (Exercise – 12B) |
| Book Name | Concise |
| Topics | Solution of Exercise-12(A), MCQs-12(A), Exercise-12(B), MCQ-12(B) and Numericals -12(B) |
| Academic Session | 2021-2022 |
Exe-12B Radioactivity ICSE Class-10 Concise Physics Selina Solutions
-: Select Exercise :-
Exercise-12(B), MCQ-12(B) and Num-12(B)
Selina Physics Solutions Radioactivity Exercise – 12 (B)
Page 306
Question 1
What do you mean by nuclear energy? What is responsible for its release?
Answer 1
Energy released by combining of nuclei of an atom or by decay of an unstable radioactive nucleus during a nuclear reaction i.e., during fusion or fission is known as nuclear energy.
Question 2
Write down the Einstein’s mass-energy equivalence relation, explaining the meaning of each symbol used in it.
Answer 2
Einstein’s mass-energy equivalence relation : E = Δmc2
Where E is the energy released due to the loss in the mass Δm and c is the speed of light.
Question 3 (Exe-12B Radioactivity ICSE Class-10 Concise)
(a) What is a.m.u ? Express 1 a.m.u. in MeV.
(b) Write the approximate mass of a proton, neutron and electron in a.m.u.
Answer 3
(a) The mass of atomic particles is expressed in terms of atomic mass unit (a.m.u.). 1 a.m.u. of mass is equivalent to 931 MeV of energy
.(b) A Mass of proton = 1.00727 a.m.u. and the Mass of neutron = 1.00865 a.m.u. and Mass of electron = 0.00055 a.m.u.
Question 4
What is nuclear fission? Name the substance used for it. Write one fission reaction.
Answer 4
Nuclear fission is the process in which a heavy nucleus is splits into two light nuclei nearly of the same size by bombarding it with slow neutrons.
![]()
Question 5
(a) Name two isotopes of uranium which are fissionable.
(b) Which of the isotope mentioned in part (a) above is easily fissionable? Give reason.
(c) State whether the neutron needed for fission reaction of the isotope mentioned in part (b) above, is slow or fast?
Answer 5
- Experimentally it is found that isotope of is more easily fissionable because the fission of is possible by sloe neutron unlike where fission is possible only by the fast neutrons.
- Slow and fast both.
Question 6
Write the approximate value of the energy released in the fission of one nucleus of ![]() . What is the reason for it?
. What is the reason for it?
Answer 6
Nearly 190 MeV of energy is released due to fission of one nucleus of ![]() . The cause of emission of this energy is the loss in mass i.e., the sum of masses of product nuclei is less than the sum of mass of the parent nucleus and neutron.
. The cause of emission of this energy is the loss in mass i.e., the sum of masses of product nuclei is less than the sum of mass of the parent nucleus and neutron.
Question 7 (Exe-12B Radioactivity ICSE Class-10 Concise)
Complete the following nuclear fission reactions.
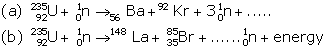
Answer 7
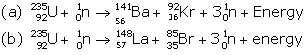
Question 8
What do you mean by the chain reaction in nuclear fission ? How is it controlled ?
Answer 8
A chain reaction is a series of nuclear fissions whereby the neutrons produced in each fission cause additional fissions, releasing enormous amount of energy.
It is controlled by absorbing some of the neutrons emitted in the fission process by means of moderators like graphite, heavy water, etc. then the energy obtained in fission can be utilized for the constructive purposes
Question 9
State two uses of nuclear fission.
Answer 9
(i) It is used in a nuclear bomb.
(ii) It is used in a nuclear reactor where the rate of release of energy is slow and controlled which is used to generate electric power.
Question 10
Give two differences between the radioactive decay and nuclear fission.
Answer 10
| Radioactive decay | Nuclear Fission |
| It is a self process. | It does not occur by itself. Neutrons are bombarded on a heavy nucleus. |
| The nucleus emits either the a or b particles
with the emission of energy in form of g rays which is not very large. |
A tremendous amount of energy is released when a heavy nucleus is bombarded with neutrons and the nucleus splits in two nearly equal fragments. |
| The rate of radioactive decay cannot be controlled. | The rate of nuclear fission can be controlled. |
Question 11
(a) What is nuclear fission? Give one example and write its nuclear reaction.
(b) What other name is given to nuclear fusion? Give reason.
Answer 11
(a) Nuclear fission is the process in which a heavy nucleus is splits into two light nuclei nearly of the same size by bombarding it with slow neutrons.
When uranium with Z = 92 is bombarded with neutron, it splits into two fragments namely barium (Z = 56) and krypton (Z = 36) and a large amount of energy is released which appears due to decrease in the mass.
![]()
(b) Nuclear fusion is also known as thermo-nuclear reaction. This is because nuclear fusion takes place at very high temperature.
Question 12 (Exe-12B Radioactivity ICSE Class-10 Concise)
Why is a very high temperature required for the process of nuclear fusion? State the approximate temperature required.
Answer 12
When two nuclei approach each other, due to their positive charge, the electrostatic force of repulsion between them becomes too strong that they do not fuse. Thus, nuclear fusion is not possible at ordinary temperature and ordinary pressure.
Hence to make the fusion possible, a high temperature of approximately 107 K and high pressure is required. At such a high temperature, due to thermal agitations both nuclei acquire sufficient kinetic energy so as to overcome the force of repulsion between them when they approach each other, and so they get fused.
Question 13
(a) Write one nuclear fusion reaction.
(b) State the approximate value of energy released in the reaction mentioned in part (a).
(c) Give reason for the release of energy stated in part (b).
Answer 13
![]()
![]()
In all three deuterium nuclei fuse to form a helium nucleus with a release of 21·6 MeV energy.
When two deuterium nuclei (![]() ) fuse, nucleus of helium isotope
) fuse, nucleus of helium isotope ![]() is formed and 3·3 MeV energy is released. This helium isotope again gets fused with one deuterium nucleus
is formed and 3·3 MeV energy is released. This helium isotope again gets fused with one deuterium nucleus![]() to form a helium nucleus and 18·3 MeV of energy is released in this process.
to form a helium nucleus and 18·3 MeV of energy is released in this process.
Question 14
Complete the following fusion reactions :
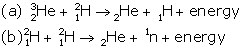
Answer 14
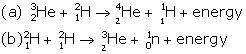
Question 15
(a) Name the process, nuclear fission or nuclear fusion, in which the energy released per unit mass is more?
(b) Name the process, fission or fusion which is possible at ordinary temperature.
Answer 15
(a) Nuclear fusion
(b) Nuclear fission
Question 16
(a) State one similarity in the process of nuclear fission or fusion
(b) State two difference between the process of nuclear fission or fusion.
Answer 16
(a) Both fission and fusion create release of neutrons and large amount of energy.
(b) Nuclear fission: A heavy nucleus splits in two nearly equal light fragments when bombarded with neutrons. It is possible at very ordinary temperature and pressure Nuclear fusion: Two light nuclei combine to form a heavy nucleus at very high temperature and high pressure. Possible only at a very high temperature (≈107 K) and a very high pressure.
Question 17
Give two examples of nuclear fusion.
Answer 17
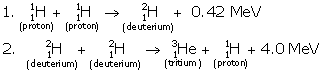
Question 18 (Exe-12B Radioactivity ICSE Class-10 Concise)
What is the source of energy of Sun or stars?
Answer 18
The source of energy in the Sun and stars is the nucleus fusion of light nuclei such as hydrogen present in them in their inner part. This takes place at a very high temperature and high pressure due to which helium nucleus is formed with the release of high amount of energy.
Question 19
Name the following nuclear reactions:
(a) ………………
(b) ……………..
Answer 19
(a) Nuclear fission
(b) Nuclear fusion
Exercise – 12(B) Multiple Choice Type , Selina ICSE Class-10 Solution Radioactivity
Page 306
Question 1
The particle used in nuclear fission for bombardment is :
(a) Alpha particle
(b) Proton
(c) Beta particle
(d) neutron
Answer 1
(d) neutron
A neutron is used in nuclear fission for bombardment.
Question 2
The temperature required for the process of nuclear fusion is nearly:
(a) 1000 K
(b) 104K
(c) 105K
(d) 107K
Answer 2
(d) 107 K
To make the fusion possible, a high temperature of approximately 107 K and high pressure is required.
Selina Physics Solutions Radioactivity, Exe -12 (B), Numericals
Page 306
Question 1
In fission of one uranium – 235 nucleus, the loss in mass is 0.2 a.m.u. Calculate the energy released.
Answer 1
1 a.m.u. = 1.66 × 10-27 kg
→ 0.2 a.m.u. = 0.2 × 1.66 × 10-27 kg
Δm = 0.332 Δ 10-27 kg
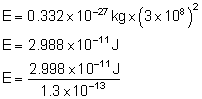
Question 2 (Exe-12B Radioactivity ICSE Class-10 Concise)
When four hydrogen nuclei combine to form a helium nucleus in the interior of sun, the loss in mass is 0.0265 a.m.u. How much energy is released ?
Answer 2
Given that Δm = 0.0265 a.m.u.
1 a.m.u. liberates 931.5 MeV of energy. Thus, energy liberated equivalent to 0.0265 a.m.u. is
= 0.0265 a.m.u. × 931.5 MeV
= 24.7 meV
Return to Concise Selina ICSE Physics Class-10
Thanks


