Language of Chemistry Exe-1C Structured Answer Chemistry Class-9 ICSE Selina Publishers Solutions Chapter-1. Step By Step ICSE Selina Concise Solutions of Chapter-1 Language of Chemistry with All Exercise including MCQs, Very Short Answer Type, Short Answer Type, Long Answer Type, Numerical and Structured/Application Questions Solved . Visit official Website CISCE for detail information about ICSE Board Class-9.
Language of Chemistry Exe-1C Structured Answer Chemistry Class-9 ICSE Concise Selina Publishers
| Board | ICSE |
| Publications | Selina Publication |
| Subject | Chemistry |
| Class | 9th |
| Chapter-1 | Language of Chemistry |
| Book Name | Concise |
| Topics | Solution of Exercise – (1C) Structured/Application/Skill Answer Type |
| Academic Session | 2023-2024 |
E. Exercise – (1C) Structured/Application/Skill Answer Type
Language of Chemistry Class-9 Chemistry Concise Solutions
Page 21
Question 1.
Elements X, Y and Z have 3, 7 and 6 electrons in their valence shells respectively. Write the formula of the compound formed between :
(a) X and Y
(b) X and Z
Answer:
(a) Valency of X is +3 and Y is -1. Formula of the compound formed between X and Y is :

∴ Chemical formula of the compound formed between X and Y is XY3
(b) Valency of X is +3 and Z is -2. Formula of the compound formed between X and Z is :
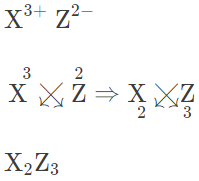
∴ Chemical formula of the compound formed between X and Z is X2Z3
Question 2.
The following figure represents the structural formula of a chemical compound.
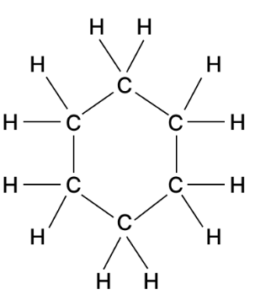
Answer the questions given below:
(a) How many Carbon and Hydrogen atoms are present in the compound.
(b) Write the molecular and empirical formulae of the compound.
(c) Calculate the percentage composition of all the elements present in the compound. [At. wt. of C = 12, H = 1]
Answer:
(a) 6 Carbon and 12 Hydrogen atoms are present.
(b) Its molecular formula is C6H12.
As the simplest ration between C and H is 1:2, hence the empirical formula is CH2
(c) Relative molecular mass of C6H12
= (6 x 12) + (1 x 12)
= 72 + 12
= 84 g
84 g of C6H12 contains 72 g of Carbon
∴ 100 g of C6H12 contains 72×100)/84
= 7200/84 = 85.7 g of Carbon.
84 g of C6H12 contains 12 g of Hydrogen
∴ 100 g of C6H12 contains 12×100)/84 g of Hydrogen
= 1200/84) = 14.3 of Hydrogen.
∴ In C6H12, C = 85.7% and H = 14.3%
— : End of Language of Chemistry Exe-1C Structured/skill Answer Class-9 ICSE Chemistry Solutions :–
Return to Return to Concise Selina ICSE Chemistry Class-9
Thanks
Please share with your friends