Language of Chemistry MCQs Class-7 Dalal Simplified ICSE Chemistry Solutions Chapter-5 Dr Viraf J Dalal Middle School Allied Publishers Solutions. Chapter-5. We Provide Step by Step Solutions of Exercise/Lesson -5 Correct answer, Give a word, Name of following, Give reasons of Dr Viraf J Dalal Middle School Chemistry Allied Publishers. Visit official Website CISCE for detail information about ICSE Board Class-7.

Language of Chemistry MCQs Class-7 Dalal Simplified ICSE Chemistry Solutions Chapter-5
| Board | ICSE |
| Class | 7th |
| Subject | Chemistry |
| Book Name | Dalal New Simplified |
| Chapter-5 | Language of Chemistry |
| Unit-1 | Language of Chemistry |
| Topic | Solution of exercise MCQs |
| Session | 2023-24 |
Objective Type Questions
Language of Chemistry MCQs Class-7 Dalal Simplified ICSE Chemistry Solutions Chapter-5
Question-1. Select the correct answer for the following options:
A: Hydrogen B: Copper [II] oxide C: Oxygen D: Copper [II] hydroxide E: Ammonia
Question: 1. The black residue obtained on heating copper carbonate.
Answer: B: Copper [II] oxide
Question: 2. The basic gas obtained when two gases react in presence of a catalyst and the reaction is exothermic.
Answer: A: Hydrogen
Question: 3. The gas obtained when potassium chlorate is heated in the presence of a catalyst.
Answer: C: Oxygen
Question: 4. The pale blue precipitated obtained when copper [II] sulphate reacts with sodium hydroxide.
Answer: D: Copper [II] hydroxide
Question: 5. The gas which reacts with oxygen to give a neutral liquid.
Answer: E: Ammonia
Question 2. Give a word equation for each of the following reactions.
Question: 1. Two solids which combine on heating, to give a liquid.
Answer: Carbon + Sulphur → Carbon disulphide
Question: 2. Two gasses which combine to give a solid.
Answer: Ammonia + Hydrogen chloride → Ammonium chloride
Question: 3. A compound which on heating shows – colour change from brown to yellow.
Answer: Lead dioxide → lead oxide + oxygen
Question: 4. A salt which reacts with aluminium hydroxide to give a reddish-brown precipitate.
Answer: Iron (II) chloride + ammonium hydroxide → ammonium chloride + iron (III) hydroxide
Question: 5. An exothermic reaction where both the reactants are gases, one of which is oxygen.
Answer: Hydrogen + Oxygen → water
Question-3. Name the following:
Question: 1. The silvery residue obtained on heating mercury oxide.
Answer: Mercury
Question: 2. The gas evolved when dilute acid is added to chalk (limestone).
Answer: Carbon dioxide
Question: 3. Two non-metal which react explosively when brought in close contact.
Answer: Iodine phosphorus
Question: 4. Two gases which react under pressure in presence of a catalyst at elevated temperatures to give a gaseous product.
Answer: Nitrogen and hydrogen
Question: 5. A catalyst which increases the rate of a chemical reaction.
Answer: Manganese dioxide
Question-4. Give reasons for the following:
Question: 1. Even though rusting is an exothermic reaction, the heat evolved when an iron nail rusts are unnoticeable.
Answer: Rusting of iron is a slow reaction and the heat evolved is very less, which is unnoticeable.
Question: 2. Electrolytic decomposition of water is a reaction involving a change of state.
Answer: Electrolytic decomposition of water is due to the passage of electric current through it.
Question: 3. A characteristic change of colour is seen when a piece of iron is added to copper sulphate solution.
Answer: When iron is added to copper [II] sulphate solution, iron displaces copper from copper sulphate and forms ferrous sulphate which is pale green coloured and there is a reddish-brown deposit on copper.
Question: 4. The catalytic oxidation of sulphur dioxide to sulphur trioxide is represented by a double arrow between reactants and products, formed in the chemical reaction.
Answer: The double arrow indicates that the reaction is reversible.
Question: 5. Silver salts are kept in dark coloured bottles.
Answer: Silver nitrate is deposited in coloured bottles because it gets decomposed by sunlight.
Question 5. Write word equations for the following chemical reactions given below. Also state the observation seen in each case.
Question: 1.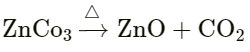
Answer: Zinc carbonate → zinc oxide + carbon dioxide Carbon dioxide gas is formed in this reaction.
Question: 2.![]()
Answer: Sodium Chloride + Silver nitrate → Silver chloride + Sodium nitrate A white precipitate (silver chloride) is formed in the above chemical reaction.
Question: 3.![]()
Answer: Sodium sulphite + hydrochloric acid → sodium chloride + water + sulphur dioxide Sulphur dioxide gas is formed in this reaction.
Question: 4.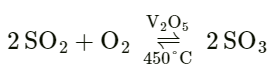
Answer: Sulphur dioxide + oxygen ⇌ Sulphur trioxide
Question: 5.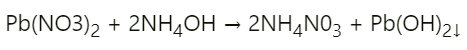
Answer: Lead nitrate + ammonium hydroxide → ammonium nitrate + lead hydroxide Lead hydroxide is a chalky white precipitate formed in this reaction.
-:End of Language of Chemistry MCQs Class-7 Dalal Simplified ICSE Chemistry Solutions:-
Return to – Dalal Simplified Chemistry for ICSE Class-7 Solutions
Thanks
Share with your friends.