Matter and Its Composition MCQs Class-7 Dalal Simplified ICSE Chemistry Solutions Chapter-1 Dr Viraf J Dalal Middle School Allied Publishers Solutions. Chapter-1. We Provide Step by Step Solutions of Exercise/Lesson -1 MCQs, Fill in the blanks, Match the following, Give reasons for the following of Dr Viraf J Dalal Middle School Chemistry Allied Publishers. Visit official Website CISCE for detail information about ICSE Board Class-7.
Matter and Its Composition MCQs Class-7 Dalal Simplified ICSE Chemistry Solutions Chapter-1
| Board | ICSE |
| Class | 7th |
| Subject | Chemistry |
| Book Name | Dalal New Simplified |
| Chapter-1 | Matter and its Composition |
| Unit-1 | Matter and its Composition |
| Topic | Solution of exercise MCQs |
| Session | 2023-24 |
Objective Type Questions
Matter and Its Composition MCQs Class-7 Dalal Simplified ICSE Chemistry Solutions Chapter-1
Question 1. Match the characteristics of the three states of matter in List I with their correct answer from List II.
| List I | List II |
|---|---|
| 1. Have no definite shape, volume or free surface | A. Solids only |
| 2. Are highly compressible and less rigid | B. Liquids only |
| 3. Have a definite volume, no definite shape and are slightly diffusible | C. Gases only |
| 4. Are not compressible and have no diffusibility | D. Liquids and gases only |
| 5. Have mass and occupy space | E. Solids, liquids and gases |
Answer 1:
| List I | List II |
|---|---|
| 1. Have no definite shape, volume or free surface | A. Liquids and gases only |
| 2. Are highly compressible and less rigid | B. Gases only |
| 3. Have a definite volume, no definite shape and are slightly diffusible | C. Liquids only |
| 4. Are not compressible and have no diffusibility | D. Solids only |
| 5. Have mass and occupy space | E. Solids, liquids and gases |
Question 2. Select the correct answer from the choice in bracket to complete each sentence:
- Matter is defined as anything that occupies __space_ and has __mass__
- The three states of matter are classified on the basis of differences of certain __physical__.
- Matter made up of one kind of particles is said to be __homogeneous__ .
- Particles in a gas possess __very large__ kinetic energy.
- The intermolecular force of attraction is _maximum_ in solids.
Question 3. Give reasons for the following.
Question: 1. Gases have no definite shape or volume.
Answer: Gases do not have a definite shape or volume because the molecules in gases are very loosely packed, they have large intermolecular spaces and hence they move around. The force of attraction between molecules is also very less, as a result gases acquire any shape or any volume.
Question: 2. Liquids have one free upper surface only.
Answer: Liquids have one free surface because they do not have a definite volume but a shape bounded by the container they are in, so the top surface of the container is the only free surface it has.
Question: 3. Globules of mercury kept in a petri dish, which is shaklen slowly, come together forming a big globule.
Answer: The formation of big globules is because of the forces of attraction existing between the molecules.
Question: 4. A crystal of iodine on slow heating in a closed flask, turns into vapours and fills the complete flask.
Answer: The particles of solid are closely packed and occupy less space while particles of gases are loosely packed and occupy the complete space available.
Question: 5. An empty tumbler lowered into a glass beaker containing water, on tilting shows bubbles of air coming out, but when not tilted, no bubbles are seen.
Answer: When the empty tumbler is tilted, the air inside the tumbler comes out and bubbles of air are seen. The air inside the tumbler was occupying space inside the beaker. When the tumbler is not tilted, the air inside does not come out, hence no bubbles are seen.
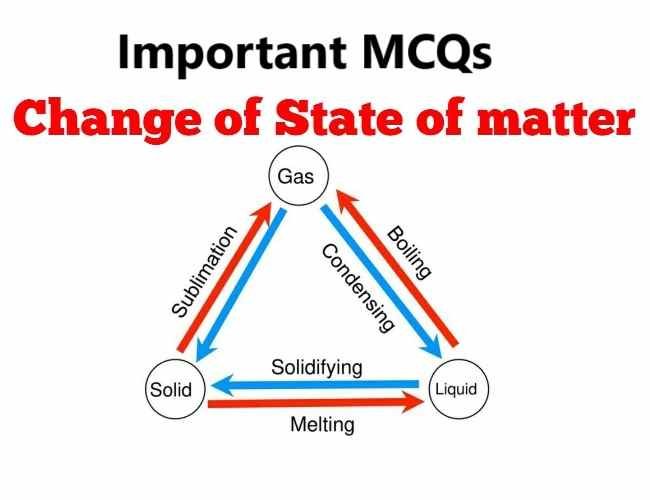
Question 4. State the correct term from A, B, C, D, E or F in List II which represents the – change of state of matter or its relevant property from List I
| List I | List II |
|---|---|
| 1. Solid ‘X’ to a liquid ‘Y’ | A. liquefaction |
| 2. Liquid ‘Y’ to its vapour ‘Z’ | B. Vaporization |
| 3. ‘Z’ to ‘Y’ | C. Melting |
| 4. ‘Y’ to ‘X | D. Solidification |
| 5. The temperature at which ‘Y’ changes to ‘Z’ | E. Melting point |
| F. Boiling point |
Answer 4:
| List I | List II |
|---|---|
| 1. Solid ‘X’ to a liquid ‘Y’ | A. Melting |
| 2. Liquid ‘Y’ to its vapour ‘Z’ | B. Vaporization |
| 3. ‘Z’ to ‘Y’ | C. liquefaction |
| 4. ‘Y’ to ‘X | D. Solidification |
| 5. The temperature at which ‘Y’ changes to ‘Z’ | E. Boiling point |
Question 5. Match the arrangement of atoms in the three states of matter in List I with the correct state in List II.
| List I | List II |
|---|---|
| 1. Arrangement of atoms, very closely packed | A. Solids |
| 2. Interparticle space is maximum | B. Liquids |
| 3. Force of attraction between particles is very weak | C. Gases |
| 4. Movement of particles is about their own position | |
| 5. Particles in the state of matter are slightly compressible and not closely packed |
Answer 5:
| List I | List II |
|---|---|
| 1. Arrangement of atoms, very closely packed | A. Solids |
| 2. Interparticle space is maximum | B. Gases |
| 3. Force of attraction between particles is very weak | C. Gases |
| 4. Movement of particles is about their own position | D. Solids |
| 5. Particles in the state of matter are slightly compressible and not closely packed | E. Liquids |
– : End of Matter and Its Composition MCQs Class-7 Dalal Simplified Solutions :–
Return to – Dalal Simplified Chemistry for ICSE Class-7 Solutions
Thanks
Share with your friends.